|
Cope Reaction
The Cope reaction or Cope elimination, developed by Arthur C. Cope, is the elimination reaction of an N-oxide to an alkene and a hydroxylamine.Typically, the amine oxide is prepared from the corresponding amine with a peroxy acid or comparable oxidant. The actual elimination requires just heat. Illustrative is a synthesis of methylenecyclohexane: Mechanism and related eliminations The reaction proceeds through the Ei pathway, with an intramolecular, cyclic 5-membered transition state. Consequently, the elimination product is always ''syn'' and rarely occurs with 6-membered rings. ( Rings with 5 or 7 or more members undergo the reaction just fine.) This organic reaction is closely related to the Hofmann elimination, but the base is a part of the leaving group. Sulfoxides can undergo an essentially identical reaction to produce sulfenic acids, which is important in the antioxidant chemistry of garlic and other ''allium''s. Selenoxides likewise undergo selenoxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arthur C
Arthur is a masculine given name of uncertain etymology. Its popularity derives from it being the name of the legendary hero King Arthur. A common spelling variant used in many Slavic, Romance, and Germanic languages is Artur. In Spanish and Italian it is Arturo. Etymology The earliest attestation of the name Arthur is in the early 9th century Welsh-Latin text '' Historia Brittonum'', where it refers to a circa 5th century Romano-British general who fought against the invading Saxons, and who later gave rise to the famous King Arthur of medieval legend and literature. A possible earlier mention of the same man is to be found in the epic Welsh poem '' Y Gododdin'' by Aneirin, which some scholars assign to the late 6th century, though this is still a matter of debate and the poem only survives in a late 13th century manuscript entitled the Book of Aneirin. A 9th-century Breton landowner named Arthur witnessed several charters collected in the '' Cartulary of Redon''. The Irish ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". In addition to pyrrolidine itself, many substituted pyrrolidines are known. Production and synthesis Industrial production Pyrrolidine is prepared industrially by the reaction of 1,4-butanediol and ammonia at a temperature of 165–200 °C and a pressure of 17–21 MPa in the presence of a cobalt- and nickel oxide catalyst, which is supported on alumina. : The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method. The catalyst is arranged as a fixed-bed and the conversion is carried out in the downflow mode. The product is obtained after m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
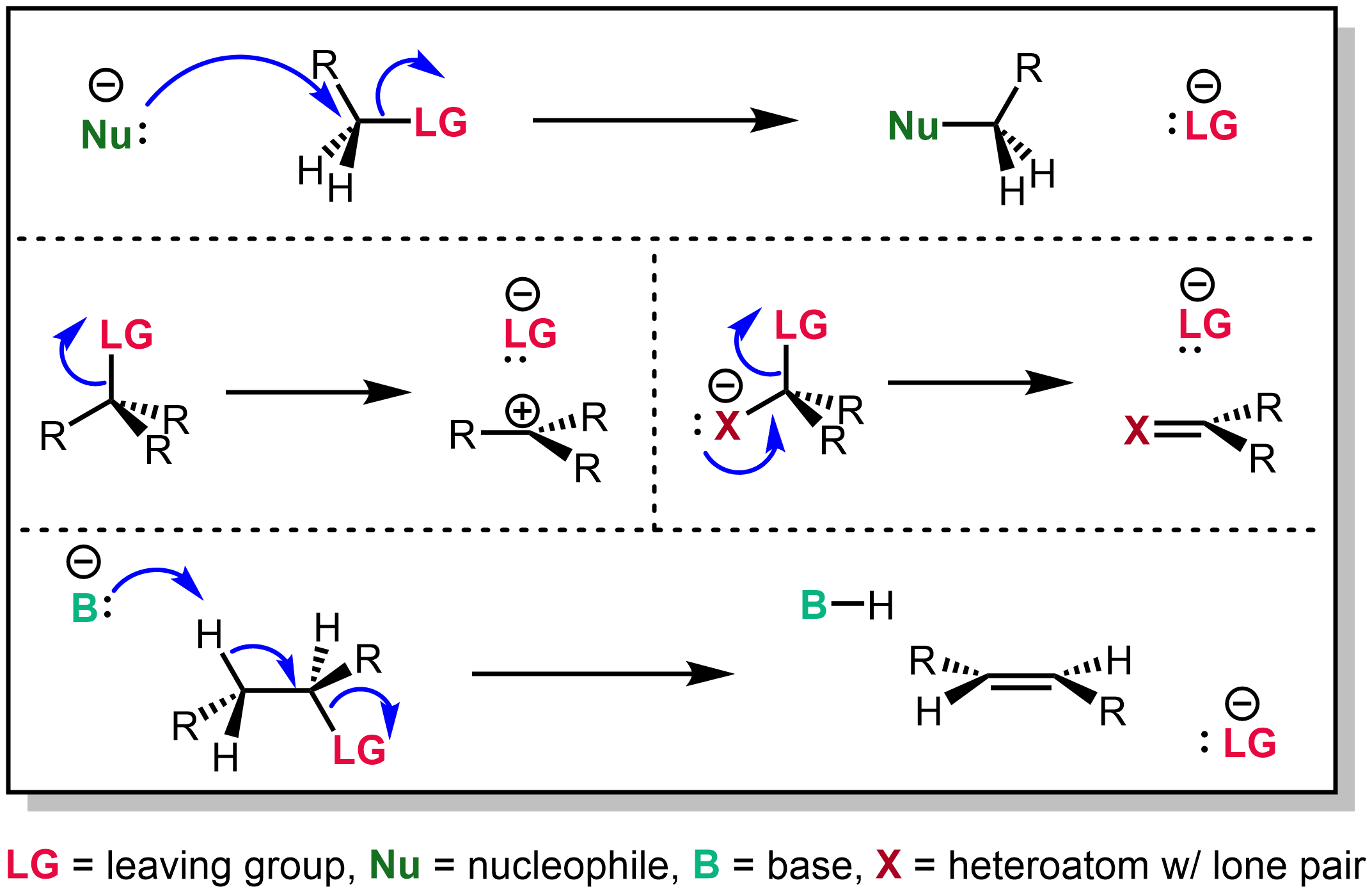
Elimination Reactions
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism. E2 mechanism The E2 mechanism, where E2 stands for bimolecular elimination, involves a one-step mechanism in which ''carbon-hydrogen'' and ''carbon-halogen'' bonds break to form a double bond (''C=C Pi bond''). The specifics of the reaction are as follows: * E2 is a single step elimination, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional group, group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides () with general structure . Oximes are usually generated by the reaction of hydroxylamine with aldehydes () or ketones (). The term ''oxime'' dates back to the 19th century, a combination of the words ''oxygen'' and ''imine''. Structure and properties If the two side-chains on the central carbon are different from each other—either an aldoxime, or a ketoxime with two different "R" groups—the oxime can often have two different geometric stereoisomeric forms according to the E/Z configuration, ''E''/''Z'' configuration. An older terminology of Descriptor (chemistry)#syn, anti, ''syn'' and ''anti'' was used to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroamination
In organic chemistry, hydroamination is the formal Addition reaction, addition of an bond of an amine across an Carbon–carbon bond, carbon-carbon multiple bond of an alkene, alkyne, diene, or allene. In the ideal case, hydroamination is Atom economy, atom economical and Green chemistry, green; and the products could see extensive use in fine-chemical, pharmaceutical, and agricultural industries. Hydroamination reactions occur spontaneously only for electrophilic alkenes and some dienes, but these are known by other names (e.g. Michael addition reaction); "hydroamination" is generally reserved for situations where the reaction requires a catalyst. Hydroamination is however of little value industrially. Hydroamination can be used Intramolecular reaction, intramolecularly to create Heterocyclic compounds, heterocycles or intermolecularly with a separate amine and unsaturated compound. File:Examples of intermolecular hydroamination.png, alt=Prototypical intermolecular hydroaminatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenoxide Elimination
Selenoxide elimination (also called α-selenation) is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction. Mechanism and stereochemistry After the development of sulfoxide elimination as an effective method for generating carbon–carbon double bonds, it was discovered that selenoxides undergo a similar process, albeit much more rapidly. Most selenoxides decompose to the corresponding alkenes at temperatures between −50 and 40 °C. Evidence suggests that the elimination is ''syn''; however, epimerization at both carbon and selenium (both of which are stereogenic) may occur during the reaction. As selenoxides can be readily prepared from nucleophilic carbonyl derivatives (enols and enolates), selenoxide elimination has grown into a general method for the preparation of α,β-unsaturated carbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenoxide
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments. Selenium can exist with oxidation state −2, +2, +4, +6. Se(II) is the dominant form in organoselenium chemistry. Down the group 16 column, the bond strength becomes increasingly weaker (234 kJ/ mol for the bond and 272 kJ/mol for the bond) and the bond lengths longer ( 198 pm, 181 pm and 141 pm). Selenium compounds are more nucleophilic than the corresponding sulfur compounds and also more acidic. The p''K''a values of are 16 for oxygen, 7 for sulfur and 3.8 for selenium. In contrast to sulfoxides, the corresponding selenoxides are unstable in the presence of β-protons and this propert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allium
''Allium'' is a large genus of monocotyledonous flowering plants with around 1000 accepted species, making ''Allium'' the largest genus in the family Amaryllidaceae and among the largest plant genera in the world. Many of the species are edible, and some have a long history of cultivation and human consumption as a vegetable including the onion, garlic, scallion, scallions, shallot, shallots, leek, leeks, and chives, with onions being the second most grown vegetable globally after tomatoes as of 2023. ''Allium'' species occur in temperate climates of the Northern Hemisphere, except for a few species occurring in Chile (such as ''A. juncifolium''), Brazil (''A. sellovianum''), and tropical Africa (Allium spathaceum, ''A. spathaceum''). They vary in height between . The flowers form an umbel at the top of a leafless stalk. The bulbs vary in size between species, from small (around 2–3 mm in diameter) to rather large (8–10 cm). Some species (such as Wels ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfenic Acid
In chemistry, a sulfenic acid is an organosulfur compound and oxoacid with the general formula . It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids () and sulfonic acids (), respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide. Properties In contrast to sulfinic and sulfonic acids, simple sulfenic acids, such as methanesulfenic acid, CH3SOH, are highly reactive and cannot be isolated in solution. In the gas phase the lifetime of methanesulfenic acid is about one minute. The gas phase structure of methanesulfenic acid was found by microwave spectroscopy (rotational spectroscopy) to be CH3–S–O–H. Sulfenic acids can be stabilized through steric effects, which prevent the sulfenic acid from condensing with itself to form thiosulfinates, RS(O)SR, such as allicin from garlic. Through the use of X-ray crystallography, the structure of such stabilized sulfenic acids were shown to be R–S–O–H. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent. Structure and bonding Sulfoxides feature relatively short S–O distances. In DMSO, the S–O distance is 1.531 Å. The sulfur center is pyramidal; the sum of the angles at sulfur is about 306°.. Sulfoxides are generally represented with the structural formula R−S(=O)−R', where R and R' are organic groups. The bond between the sulfur and oxygen atoms is intermediate of a dative bond and a polarized double bond. The double-bond resonance form implies 10 electrons around sulfur (10-S-3 in N-X-L notation). The double-bond character of the S−O bond may be accou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaving Group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving group'' is a less formal but more commonly used synonym of the term ''nucleofuge''; although IUPAC gives the term a broader definition. A species' ability to serve as a leaving group can affect whether a reaction is viable, as well as what mechanism the reaction takes. Leaving group ability depends strongly on context, but often correlates with ability to stabilize additional electron density from bond heterolysis. Common anionic leaving groups are , and halides and sulfonate esters such as tosylate (). Water (), alcohols (), and amines () are common neutral leaving groups, although they often require activating catalysts. Some moieties, such as hydride (H−) serve as leaving groups only extremely rarely. Nomenclature IUPAC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word "base": '' Arrhenius bases'', '' Brønsted bases'', and '' Lewis bases''. All definitions agree that bases are substances that react with acid An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...s, as originally proposed by Guillaume-François Rouelle, G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form hydroxide ions OH−. These ions can react with Hydron (chemistry), hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Calcium hydroxide, Ca(OH)2. Such aqueous hydroxide solutions were also described by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |