|
Compartment (chemistry)
In chemistry, a compartment is a part of a protein that serves a specific function. They are essentially protein subunits with the added condition that a compartment has distinct functionality, rather than being just a structural component. There may be multiple compartments on one and the same protein. One example is the case of pyruvate dehydrogenase complex. This is the enzyme which catalyses pyruvate decarboxylation, the reaction of pyruvate with coenzyme A Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric ac ... and the major entry point into the TCA cycle: :Pyruvate + Coenzyme A + NAD+ ⇒ acetyl-CoA + NADH + H+ + CO2 Pyruvate dehydrogenase has three chemical compartments; E1 ( pyruvate decarboxylase), E2 ( dihydrolipoyl transacetylase) and E3 ( dihydrolipoyl dehydrogenase). Eac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Subunit
In structural biology, a protein subunit is a polypeptide chain or single protein molecule that assembles (or "''coassembles''") with others to form a protein complex. Large assemblies of proteins such as viruses often use a small number of types of protein subunits as building blocks. A subunit is often named with a Greek or Roman letter, and the numbers of this type of subunit in a protein is indicated by a subscript. For example, ATP synthase has a type of subunit called α. Three of these are present in the ATP synthase molecule, leading to the designation α3. Larger groups of subunits can also be specified, like α3β3-hexamer and c-ring. Naturally occurring proteins that have a relatively small number of subunits are referred to as oligomeric.Quote: ''Oligomer molecule: A molecule of intermediate relative molecular mass, the structure of which essentially comprises a small plurality of units derived, actually or conceptually, from molecules of lower relative molecula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
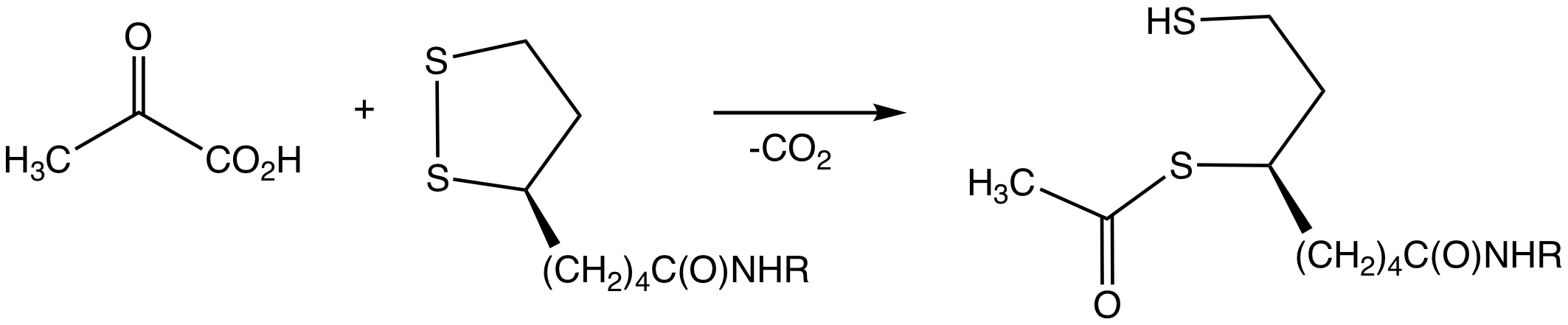
Pyruvate Dehydrogenase Complex
Pyruvate dehydrogenase complex (PDC) is a complex of three enzymes that converts pyruvate into acetyl-CoA by a process called pyruvate decarboxylation. Acetyl-CoA may then be used in the citric acid cycle to carry out cellular respiration, and this complex links the glycolysis metabolic pathway to the citric acid cycle. Pyruvate decarboxylation is also known as the "pyruvate dehydrogenase reaction" because it also involves the oxidation of pyruvate. The levels of pyruvate dehydrogenase enzymes play a major role in regulating the rate of carbohydrate metabolism and are strongly stimulated by the evolutionarily ancient hormone insulin. The PDC is opposed by the activity of pyruvate dehydrogenase kinase, and this mechanism plays a pivotal role in regulating rates of carbohydrate and lipid metabolism in many physiological states across taxa, including feeding, starvation, diabetes mellitus, hyperthyroidism, and hibernation. The multi-enzyme complex is related structurally and fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyruvate Decarboxylation
Pyruvate decarboxylation or pyruvate oxidation, also known as the link reaction (or oxidative decarboxylation of pyruvate), is the conversion of pyruvate into acetyl-CoA by the enzyme complex pyruvate dehydrogenase complex. The reaction may be simplified as: :Pyruvate + NAD+ + CoA → Acetyl-CoA + NADH + CO2 Pyruvate oxidation is the step that connects glycolysis and the Krebs cycle. In glycolysis, a single glucose molecule (6 carbons) is split into 2 pyruvates (3 carbons each). Because of this, the link reaction occurs twice for each glucose molecule to produce a total of 2 acetyl-CoA molecules, which can then enter the Krebs cycle. Energy-generating ions and molecules, such as amino acids and carbohydrates, enter the Krebs cycle as acetyl coenzyme A and oxidize in the cycle. The pyruvate dehydrogenase complex (PDC) catalyzes the decarboxylation of pyruvate, resulting in the synthesis of acetyl-CoA, CO2, and NADH. In eukaryotes, this enzyme complex regulates pyruvate met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a Substrate (chemistry), substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenic acid, pantothenate (vitamin B5), and adenosine triphosphate (ATP). In acetyl-CoA, its acetyl form, coenzyme A is a highly versatile molecule, serving metabolic functions in both the Anabolism, anabolic and Catabolism, catabolic pathways. Acetyl-CoA is utilised in the post-translational regulation and allosteric regulation of pyruvate dehydrogenase and carboxylase to maintain and support the partition of Pyruvic acid, pyruvate synthesis and degradation. Discovery of structure Coenzyme A was ident ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TCA Cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of chemical reaction, biochemical reactions that release the energy stored in nutrients through acetyl-CoA Redox, oxidation. The energy released is available in the form of Adenosine triphosphate, ATP. The Hans Krebs (biochemist), Krebs cycle is used by organisms that generate energy via Cellular respiration, respiration, either anaerobic respiration, anaerobically or aerobic respiration, aerobically (organisms that Fermentation, ferment use different pathways). In addition, the cycle provides precursor (chemistry), precursors of certain amino acids, as well as the reducing agent nicotinamide adenine dinucleotide, NADH, which are used in other reactions. Its central importance to many Metabolic pathway, biochemical pathways suggests that it was one of the earliest metabolism components. Even though it is branded as a "cycle", it is not necessa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyruvate Dehydrogenase
Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide. The conversion requires the coenzyme thiamine pyrophosphate. Pyruvate dehydrogenase is usually encountered as a component, referred to as E1, of the pyruvate dehydrogenase complex (PDC). PDC consists of other enzymes, referred to as E2 and E3. Collectively E1-E3 transform pyruvate, NAD+, coenzyme A into acetyl-CoA, CO2, and NADH. The conversion is crucial because acetyl-CoA may then be used in the citric acid cycle to carry out cellular respiration. To distinguish between this enzyme and the PDC, it is systematically called pyruvate dehydrogenase (acetyl-transferring). Mechanism The thiamine pyrophosphate (TPP) converts to an ylide by deprotonation. The ylide attack the ketone group of pyruvate. The resulting adduct decarboxylates. The resulting 1,3-dipole reductively acetylates lipoamide-E2. In terms of details, biochem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyruvate Decarboxylase
Pyruvate decarboxylase is an enzyme () that catalyses the decarboxylation of pyruvic acid to acetaldehyde. It is also called 2-oxo-acid carboxylase, alpha-ketoacid carboxylase, and pyruvic decarboxylase. In anaerobic conditions, this enzyme participates in the fermentation process that occurs in yeast, especially of the genus ''Saccharomyces'', to produce ethanol by fermentation. It is also present in some species of fish (including goldfish and carp) where it permits the fish to perform ethanol fermentation (along with lactic acid fermentation) when oxygen is scarce. Pyruvate decarboxylase starts this process by converting pyruvate into acetaldehyde and carbon dioxide. Pyruvate decarboxylase depends on cofactors thiamine pyrophosphate (TPP) and magnesium. This enzyme should not be mistaken for the unrelated enzyme pyruvate dehydrogenase, an oxidoreductase (), that catalyzes the oxidative decarboxylation of pyruvate to acetyl-CoA. Structure Pyruvate decarboxylase occurs a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrolipoyl Transacetylase
Dihydrolipoyl transacetylase (or dihydrolipoamide acetyltransferase) is an enzyme component of the multienzyme pyruvate dehydrogenase complex. The pyruvate dehydrogenase complex is responsible for the pyruvate decarboxylation step that links glycolysis to the citric acid cycle. This involves the transformation of pyruvate from glycolysis into acetyl-CoA which is then used in the citric acid cycle to carry out cellular respiration. There are three different enzyme components in the pyruvate dehydrogenase complex. Pyruvate dehydrogenase (EC 1.2.4.1) is responsible for the oxidation of pyruvate, dihydrolipoyl transacetylase (this enzyme; EC 2.3.1.12) transfers the acetyl group to coenzyme A (CoA), and dihydrolipoyl dehydrogenase (EC 1.8.1.4) regenerates the lipoamide. Because dihydrolipoyl transacetylase is the second of the three enzyme components participating in the reaction mechanism for conversion of pyruvate into acetyl CoA, it is sometimes referred to as E2. In humans, dihydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrolipoyl Dehydrogenase
Dihydrolipoamide dehydrogenase (DLD), also known as dihydrolipoyl dehydrogenase, mitochondrial, is an enzyme that in humans is encoded by the ''DLD'' gene. DLD is a flavoprotein enzyme that oxidizes dihydrolipoamide to lipoamide. Dihydrolipoamide dehydrogenase (DLD) is a mitochondrial enzyme that plays a vital role in energy metabolism in eukaryotes. This enzyme is required for the complete reaction of at least five different multi-enzyme complexes. Additionally, DLD is a flavoenzyme oxidoreductase that contains a reactive disulfide bridge and a FAD cofactor that are directly involved in catalysis. The enzyme associates into tightly bound homodimers required for its enzymatic activity. File:Lipoamide-2D-skeletal.png, Lipoamide File:Dihydrolipoamide.svg, Dihydrolipoamide Structure The protein encoded by the DLD gene comes together with another protein to form a dimer in the central metabolic pathway. Several amino acids within the catalytic pocket have been identified a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |