|
Cobalt(III) Nitrate
Cobalt(III) nitrate is an inorganic compound with the chemical formula Co(NO3)3.W. Levason and C. A. McAuliffe (1974): "Higher oxidation state chemistry of iron, cobalt, and nickel". ''Coordination Chemistry Reviews'', volume 12, issue 2, pages 151-184. It is a green, diamagnetic solid that sublimes at ambient temperature. Structure The compound is a molecular coordination complex. The three bidentate nitrate ligands give a distorted octahedral arrangement. The nitrate ligands are planar. With D3 symmetry, the molecule is chiral. The Co-O bond lengths are about 190 pm long. The O-Co-O angles for the two oxygens in the same nitrate is about 68 degrees. The same geometry seems to persist in carbon tetrachloride solution.R. J. Fereday, N. Logan and D. Sutton (1969): "Anhydrous cobalt(III) nitrate: preparation, spectra, and reactions with some organic ligands". ''Journal of the Chemical Society A: Inorganic, Physical, Theoretical'', volume 1969, issue 0, pages 2699-2703. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt(III) Fluoride
Cobalt(III) fluoride is the inorganic compound with the formula . Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds. The related cobalt(III) chloride is also known but is extremely unstable.Arthur W. Chester, El-Ahmadi Heiba, Ralph M. Dessau, and William J. Koehl Jr. (1969): "The interaction of cobalt(III) with chloride ion in acetic acid". ''Inorganic and Nuclear Chemistry Letters'', volume 5, issue 4, pages 277-283. Cobalt(III) bromide and cobalt(III) iodide have not been synthesized. Structure Anhydrous Anhydrous cobalt trifluoride crystallizes in the rhombohedral group, specifically according to the aluminium trifluoride motif, with ''a'' = 527.9 pm, ''α'' = 56.97°. Each cobalt atom is bound to six fluorine atoms in octahedral geometry, with Co–F distances of 189 pm. Each fluoride is a doubly bridging ligand. Hydrates A hydrate is known. It is conjectured to be better described as . T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III) Oxalate
Ferric oxalate, also known as iron(III) oxalate, is a chemical compound composed of ferric ions and oxalate ligands; it may also be regarded as the ferric salt of oxalic acid. The anhydrous material is pale yellow; however, it may be hydrated to form several hydrates, such as potassium ferrioxalate, or , which is bright green in colour. Structure Tetrahydrate The crystal structure of the tetrahydrate was determined in 2015. It has a triclinic unit cell containing two iron atoms. Each iron atom has octahedral coordination bonds to the oxygen atoms of three oxalate molecules and one water molecule. Two of those three oxalates, lying in approximately perpendicular planes, are tetradentate, and connect the iron atoms into zigzag chains. The third oxalate molecule is bidentate, and connects iron atoms of adjacent chains, creating an open-layered structure. Half of the water molecules lie, unbound, between those chains. Mössbauer spectrum of indicates that iron is present in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III) Nitrate
Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts. Hydrates Iron(III) nitrate is deliquescent, and it is commonly found as the nonahydrate Fe(NO3)3· 9H2O, which forms colourless to pale violet crystals. This compound is the trinitrate salt of the aquo complex e(H2O)6sup>3+. Other hydrates ·''x'', include: * tetrahydrate (''x''=4), more precisely triaqua dinitratoiron(III) nitrate monohydrate, ·, has complex cations where atom is coordinated with two nitrate anions as bidentate ligands and three of the four water molecules, in a pentagonal bipyramid configuration with two water molecules at the poles.H. Schmidt, A. Asztalos, F. Bok and W. Voigt (2012): "New iron(III) nitrate hydrates: ·''x'' with ''x'' = 4, 5 and 6". ''Acta Crystallographica Section C - Inorganic Compounds'', volume ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt(III) Hydroxide
Cobalt(III) hydroxide or cobaltic hydroxide is a chemical compound with formula or . It is an ionic compound, with trivalent cobalt cations and hydroxyl anions . The compound is known in two structurally different forms, "brownish-black" and "green". The brownish-black form is a stable solid and can be prepared by reaction of water solutions of cobalt(II) chloride and sodium hydroxide, followed by oxidation with ozone.Pankratov, D.A., Veligzhanin, A.A., and Zubavichus, Y.V. (2013): "Structural Features of Green Cobalt(III) Hydroxide". ''Russian Journal of Inorganic Chemistry'', volume 58, issue 1, pages 67–73. The green form, formerly thought to be cobalt(II) peroxide, apparently requires carbon dioxide as a catalyst. It can be prepared by adding hydrogen peroxide to a solution of cobalt(II) chloride in 96% ethanol at –30 to –35°C, then adding a 15% solution of sodium carbonate in water with intense stirring. The resulting dark green powder is fairly stable at liqu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt(III) Chloride
Cobalt(III) chloride or cobaltic chloride is an unstable and elusive compound of cobalt and chlorine with formula . In this compound, the cobalt atoms have a formal charge of +3.Arthur W. Chester, El-Ahmadi Heiba, Ralph M. Dessau, and William J. Koehl Jr. (1969): "The interaction of cobalt(III) with chloride ion in acetic acid". ''Inorganic and Nuclear Chemistry Letters'', volume 5, issue 4, pages 277-283. The compound has been reported to exist in the gas phase at high temperatures, in equilibrium with cobalt(II) chloride and chlorine gas.W. D. Halstead (1975): "A review of saturated vapour pressures and allied data for the principal corrosion products of iron, chromium, nickel and cobalt in flue gases". ''Corrosion Science'', volume 15, issues 6–12, pages 603-625. It has also been found to be stable at very low temperatures, dispersed in a frozen argon matrix.David W. Green, Dana P. McDermott, and Adelle Bergman (1983): "Infrared spectra of the matrix-isolated chlorides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt(III) Fluoride
Cobalt(III) fluoride is the inorganic compound with the formula . Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds. The related cobalt(III) chloride is also known but is extremely unstable.Arthur W. Chester, El-Ahmadi Heiba, Ralph M. Dessau, and William J. Koehl Jr. (1969): "The interaction of cobalt(III) with chloride ion in acetic acid". ''Inorganic and Nuclear Chemistry Letters'', volume 5, issue 4, pages 277-283. Cobalt(III) bromide and cobalt(III) iodide have not been synthesized. Structure Anhydrous Anhydrous cobalt trifluoride crystallizes in the rhombohedral group, specifically according to the aluminium trifluoride motif, with ''a'' = 527.9 pm, ''α'' = 56.97°. Each cobalt atom is bound to six fluorine atoms in octahedral geometry, with Co–F distances of 189 pm. Each fluoride is a doubly bridging ligand. Hydrates A hydrate is known. It is conjectured to be better described as . T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three isotopes occur naturally, C and C being stable, while C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity. Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of Carbon-based life, all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
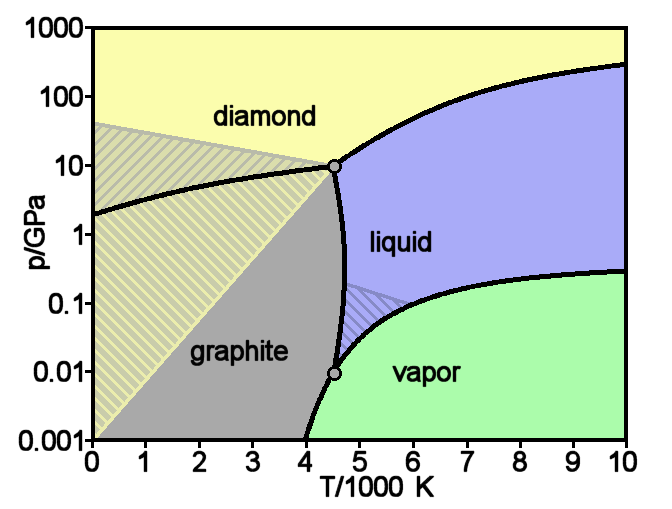
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular cry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitrogen Pentoxide
Dinitrogen pentoxide is the chemical compound with the formula , also known as nitrogen pentoxide or nitric anhydride. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that melt at 41 °C. Its boiling point is 47 °C, and sublimes slightly above room temperature, yielding a colorless gas.Peter Steele Connell The Photochemistry of Dinitrogen Pentoxide'. Ph. D. thesis, Lawrence Berkeley National Laboratory. Dinitrogen pentoxide is an unstable and potentially dangerous oxidizer that once was used as a reagent when dissolved in chloroform for nitrations but has largely been superseded by nitronium tetrafluoroborate (). is a rare example of a compound that adopts two structures depending on the conditions. The solid is a salt, nitronium nitrate, consisting of separate nitronium cations and nitrate anions ; but in the gas phase and under some other conditions it is a covalently ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon ( graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |