|
Cloxacillin
Cloxacillin is an antibiotic useful for the treatment of a number of bacterial infections. This includes impetigo, cellulitis, pneumonia, septic arthritis, and otitis externa. It is not effective for methicillin-resistant ''Staphylococcus aureus'' (MRSA). It is used by mouth and by injection. Side effects include nausea, diarrhea, and allergic reactions including anaphylaxis. ''Clostridium difficile'' diarrhea may also occur. It is not recommended in people who have previously had a penicillin allergy. Use during pregnancy appears to be relatively safe. Cloxacillin is in the penicillin family of medications. Cloxacillin was patented in 1960 and approved for medical use in 1965. It is on the World Health Organization's List of Essential Medicines. It is not commercially available in the United States. Mechanism of action It is semisynthetic and in the same class as penicillin. Cloxacillin is used against staphylococci that produce beta-lactamase, due to its large R chain, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flucloxacillin
Flucloxacillin, also known as floxacillin, is an antibiotic used to treat skin infections, external ear infections, infections of leg ulcers, diabetic foot infections, and infection of bone. It may be used together with other medications to treat pneumonia, and endocarditis. It may also be used prior to surgery to prevent ''Staphylococcus'' infections. It is not effective against methicillin-resistant ''Staphylococcus aureus'' (MRSA). It is taken by mouth or given by injection into a vein or muscle. Common side effects include an upset stomach. Other side effects may include muscle or joint pains, shortness of breath, and liver problems. It appears to be safe during pregnancy and breastfeeding. It should not be used in those who are allergic to penicillin. It is a narrow-spectrum beta-lactam antibiotic of the penicillin class. It is similar in effect to cloxacillin and dicloxacillin, being active against penicillinase forming bacteria. Flucloxacillin was patented in 196 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicloxacillin
Dicloxacillin is a narrow-spectrum β-lactam antibiotic of the penicillin class. It is used to treat infections caused by susceptible (non-resistant) Gram-positive bacteria.Product Information: DICLOXACILLIN SODIUM-dicloxacillin sodium capsule. Teva Pharmaceuticals USA Inc, Revised 8/2015 It is active against beta-lactamase-producing organisms such as ''Staphylococcus aureus'', which would otherwise be resistant to most penicillins. Dicloxacillin is available under a variety of trade names including Diclocil ( BMS). It was patented in 1961 and approved for medical use in 1968. Medical uses Dicloxacillin is used to treat mild-to-moderate staphylococcal infections. To decrease the development of resistance, dicloxacillin is recommended to treat infections that are suspected or proven to be caused by beta-lactamase-producing bacteria. Dicloxacillin is similar in pharmacokinetics, antibacterial activity, and indications to flucloxacillin, and the two agents are considered interc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxacillin
Oxacillin (trade name Bactocill) is a narrow-spectrum antibiotic, narrow-spectrum beta-lactam antibiotic of the penicillin class developed by Beecham (pharmaceutical company), Beecham. It was patented in 1960 and approved for medical use in 1962. Medical uses Oxacillin is a penicillinase-resistant β-lactam. It is similar to methicillin, and has replaced methicillin in clinical use. Other related compounds are nafcillin, cloxacillin, dicloxacillin, and flucloxacillin. Since it is resistant to penicillinase enzymes, such as that produced by ''Staphylococcus aureus'', it is widely used clinically in the US to treat penicillin-resistant ''Staphylococcus aureus''. However, with the introduction and widespread use of both oxacillin and methicillin, antibiotic resistance, antibiotic-resistant strains called Methicillin-resistant Staphylococcus aureus, methicillin-resistant and oxacillin-resistant ''Staphylococcus aureus'' (MRSA/ORSA) have become increasingly prevalent worldwide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillins
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from ''Penicillium'' moulds, principally ''P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G (intramuscular or intravenous use) and penicillin V (given by mouth). Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use. 10% of the population claims penicillin allergies but because the frequency of positive skin test results decreases by 10% with each year of avoidance, 90% of these patients can tolerate penicillin. Additionally, those with peni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from ''Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G ( intramuscular or intravenous use) and penicillin V (given by mouth). Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use. 10% of the population claims penicillin allergies but because the frequency of positive skin test results decreases by 10% with each year of avoidance, 90% of these patients can tolerate penicillin. Additionally, those w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WHO Model List Of Essential Medicines
The WHO Model List of Essential Medicines (aka Essential Medicines List or EML), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health system. The list is frequently used by countries to help develop their own local lists of essential medicines. , more than 155 countries have created national lists of essential medicines based on the World Health Organization's model list. This includes both developed and developing countries. The list is divided into core items and complementary items. The core items are deemed to be the most cost-effective options for key health problems and are usable with little additional health care resources. The complementary items either require additional infrastructure such as specially trained health care providers or diagnostic equipment or have a lower cost–benefit ratio. About 25% of items are in the complementary list. Some medica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoxazoles
Isoxazole is an electron-rich azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring. Isoxazolyl is the univalent radical derived from isoxazole. Occurrence Isoxazole rings are found in some natural products, such as ibotenic acid and muscimol. Synthesis Isoxazole can be synthesised via a variety of methods. Examples include via a 1,3-dipolar cycloaddition of nitrile oxides with alkynes; or the reaction of hydroxylamine with 1,3-diketones or derivatives of propiolic acid. Photochemistry The photolysis of isoxazole was first reported in 1966. Due to the weak N-O bond, the isoxazole ring tends to collapse under UV irradiation, rearranging to oxazole through azirine intermediate. Meanwhile, the azirine intermediate can react with nucleophiles, especially carboxylic acids. Given the photoreactions, isoxazole group is developed as a native photo-cross-linker for photoaffinity labeling and chemoproteomic studies. Pharmaceuticals and h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
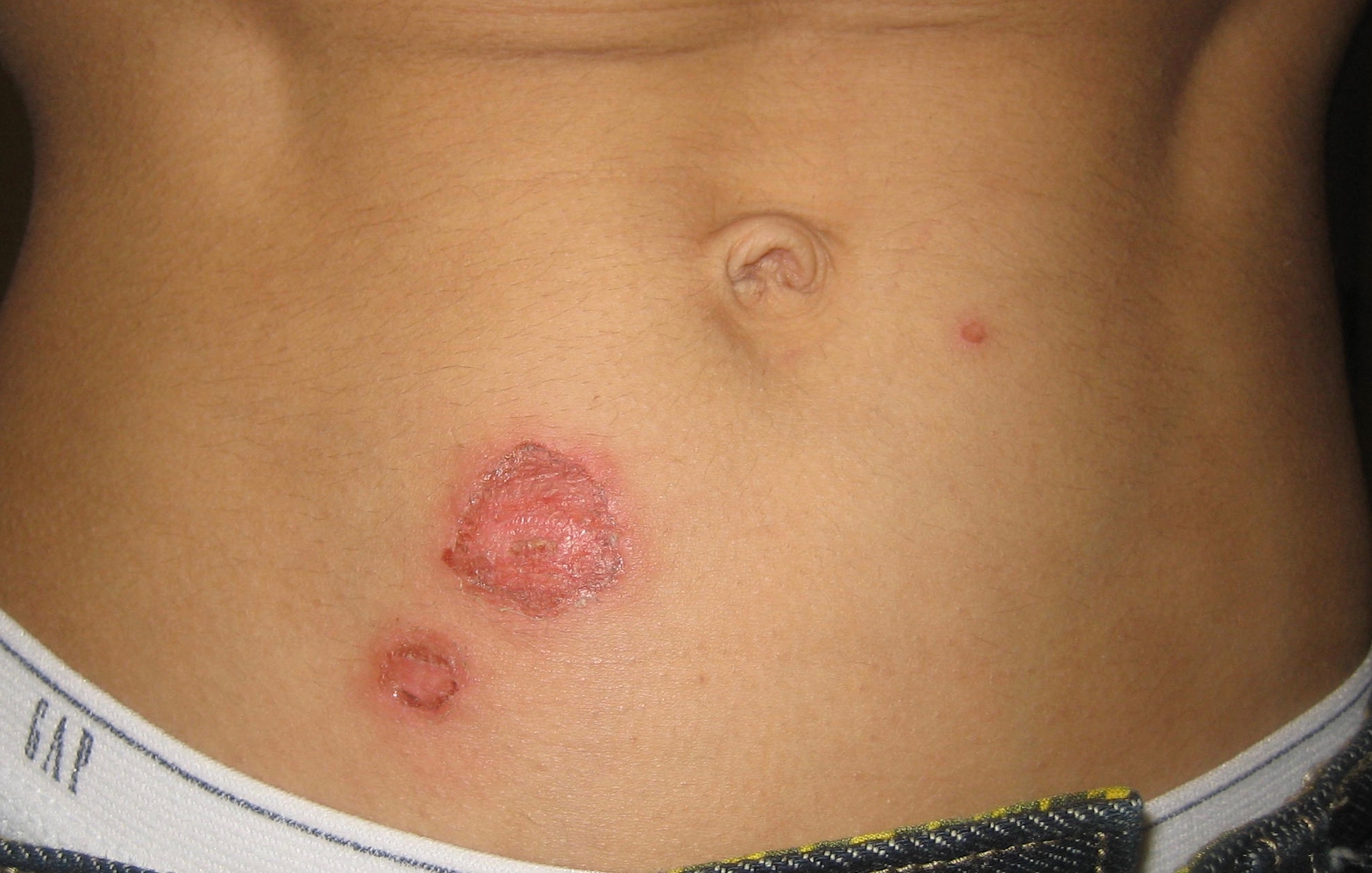
Impetigo
Impetigo is a bacterial infection that involves the superficial skin. The most common presentation is yellowish crusts on the face, arms, or legs. Less commonly there may be large blisters which affect the groin or armpits. The lesions may be painful or itchy. Fever is uncommon. It is typically due to either ''Staphylococcus aureus'' or ''Streptococcus pyogenes''. Risk factors include attending day care, crowding, poor nutrition, diabetes mellitus, contact sports, and breaks in the skin such as from mosquito bites, eczema, scabies, or herpes. With contact it can spread around or between people. Diagnosis is typically based on the symptoms and appearance. Prevention is by hand washing, avoiding people who are infected, and cleaning injuries. Treatment is typically with antibiotic creams such as mupirocin or fusidic acid. Antibiotics by mouth, such as cefalexin, may be used if large areas are affected. Antibiotic-resistant forms have been found. Impetigo affected ab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beecham (pharmaceutical Company)
The Beecham Group plc was a British pharmaceutical company. It was once a constituent of the FTSE 100 Index. Beecham, after having merged with American pharmaceutical company SmithKline Beckman to become SmithKline Beecham, merged with Glaxo Wellcome to become GlaxoSmithKline (GSK). GSK still uses the Beechams brand name in the UK for its over-the-counter cold and flu relief products. Early history Beecham began as the family business of Thomas Beecham (1820–1907), a chemist. (Beecham would become the grandfather of music conductor Thomas Beecham, 1879–1961). As a boy, Beecham worked as a shepherd, selling herbal remedies as a sideline. He later became a travelling salesman or peddler full time. His first product was Beecham's Pills, a laxative, in 1842. Subsequent success enabled him to open a shop in Wigan in 1847. Beecham opened its first factory in 1849 in St Helens, Lancashire, for the rapid production of medicines. Under his son, Sir Joseph Beecham, 1st Baronet (1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-lactamases
Beta-lactamases, (β-lactamases) are enzymes () produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems ( ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties. Beta-lactam antibiotics are typically used to target a broad spectrum of gram-positive and gram-negative bacteria. Beta-lactamases produced by gram-negative bacteria are usually secreted, especially when antibiotics are present in the environment. Structure The structure of a ''Streptomyces'' serine β-lactamase (SBLs) is given by . The alpha-beta fol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin Allergy
The side effects of penicillin are bodily responses to penicillin and closely related antibiotics that do not relate directly to its effect on bacteria. A side effect is an effect that is not intended with normal dosing. Some of these reactions are visible and some occur in the body's organs or blood. Penicillins are a widely used group of medications that are effective for the treatment of a wide variety of bacterial infections in human adults and children as well as other species. Some side effects are predictable, of which some are common but not serious, some are uncommon and serious and others are rare. The route of administration of penicillin can have an effect on the development of side effects. An example of this is irritation and inflammation that develops at a peripheral infusion site when penicillin is administered intravenously. In addition, penicillin is available in different forms. There are different penicillin medications ( penicillin G benzathine, penicillin G pot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellulitis
Cellulitis is usually a bacterial infection involving the inner layers of the skin. It specifically affects the dermis and subcutaneous fat. Signs and symptoms include an area of redness which increases in size over a few days. The borders of the area of redness are generally not sharp and the skin may be swollen. While the redness often turns white when pressure is applied, this is not always the case. The area of infection is usually painful. Lymphatic vessels may occasionally be involved, and the person may have a fever and feel tired. The legs and face are the most common sites involved, although cellulitis can occur on any part of the body. The leg is typically affected following a break in the skin. Other risk factors include obesity, leg swelling, and old age. For facial infections, a break in the skin beforehand is not usually the case. The bacteria most commonly involved are streptococci and ''Staphylococcus aureus''. In contrast to cellulitis, erysipelas is a b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |