|
Cinnamoyl-CoA
Cinnamoyl-coenzyme A is an intermediate in the Phenylpropanoids metabolism, phenylpropanoid metabolic pathway. Enzymes using cinnamoyl-coenzyme A * Cinnamoyl-CoA reductase, an enzyme that catalyzes the chemical reaction cinnamaldehyde + CoA + NADP+ → cinnamoyl-CoA + NADPH + H+ * Pinosylvin synthase, an enzyme that catalyzes the chemical reaction 3 malonyl-CoA + cinnamoyl-CoA → 4 CoA + pinosylvin + 4 CO2 * Cinnamoyl-CoA:phenyllactate CoA-transferase, an enzyme that catalyzes the chemical reaction (E)-cinnamoyl-CoA + (R)-phenyllactate → (E)-cinnamate + (R)-phenyllactyl-CoA References Thioesters of coenzyme A Cinnamate esters {{aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamoyl-CoA Reductase
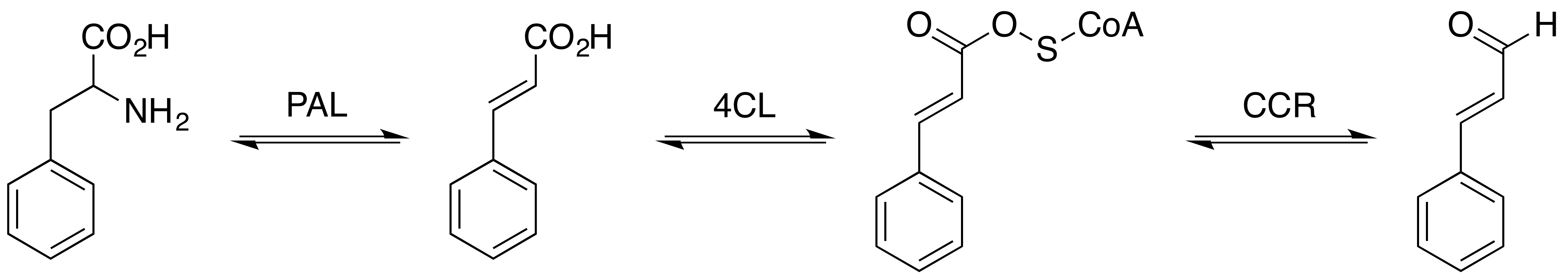
Cinnamoyl-CoA reductase (), Systematic name, systematically named cinnamaldehyde:NADP+ oxidoreductase (CoA-cinnamoylating) but commonly referred to by the acronym CCR, is an enzyme that catalysis, catalyzes the Redox, reduction of a substituted cinnamoyl-CoA to its corresponding cinnamaldehyde, utilizing nicotinamide adenine dinucleotide phosphate, NADPH and hydrogen ion, H+ and releasing free coenzyme A, CoA and nicotinamide adenine dinucleotide phosphate, NADP+ in the process. Common biologically relevant cinnamoyl-CoA Substrate (chemistry), substrates for CCR include P-Coumaroyl-CoA, ''p''-coumaroyl-CoA and feruloyl-CoA, which are converted into ''p''-coumaraldehyde and Coniferyl aldehyde, coniferaldehyde, respectively, though most CCRs show activity toward a variety of other substituted cinnamoyl-CoA's as well. Catalyzing the first committed step in monolignol biosynthesis, this enzyme plays a critical role in lignin formation, a process important in plants both for structural de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula or . Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus '' Cinnamomum''. It is an essential oil. The bark of cinnamon tree contains high concentrations of cinnamaldehyde. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is '' trans''-cinnamaldehyde. The molecule consists of a benzene ring attached to an unsaturated aldehyde. Cinnamaldehyde is an α,β-unsaturated carbonyl compound. Its color is due to the π → π* transition: increased conjugation in comparison with acrolein shifts this band towa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylpropanoids Metabolism
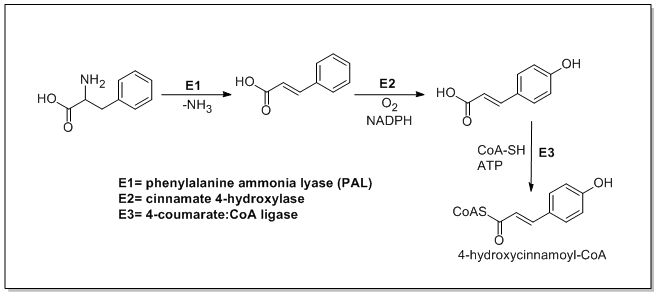
The biosynthesis of phenylpropanoids involves a number of enzymes. From amino acids to cinnamates In plants, all phenylpropanoids are derived from the amino acids phenylalanine and tyrosine. Phenylalanine ammonia-lyase (PAL, a.k.a. phenylalanine/tyrosine ammonia-lyase) is an enzyme that transforms L-phenylalanine and tyrosine into trans-cinnamic acid and p-coumaric acid, ''p''-coumaric acid, respectively. Trans-cinnamate 4-monooxygenase (cinnamate 4-hydroxylase) is the enzyme that transforms trans-cinnamate into 4-hydroxycinnamate (''p''-coumaric acid). 4-Coumarate-CoA ligase is the enzyme that transforms 4-coumarate (''p''-coumaric acid) into 4-coumaroyl-CoA. Enzymes associated with biosynthesis of hydroxycinnamic acids * Cinnamyl-alcohol dehydrogenase (CAD), an enzyme that transforms cinnamyl alcohol into cinnamaldehyde * Sinapine esterase, an enzyme that transforms sinapoylcholine into sinapate (sinapic acid) and choline * Trans-cinnamate 2-monooxygenase, an enzyme that tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pinosylvin Synthase
In enzymology, a pinosylvin synthase () is an enzyme that catalyzes the chemical reaction :3 malonyl-CoA + cinnamoyl-CoA \rightleftharpoons 4 CoA + pinosylvin + 4 CO2 Thus, the two substrates of this enzyme are malonyl-CoA and cinnamoyl-CoA, whereas its 3 products are CoA, pinosylvin, and CO2. This enzyme belongs to the family of transferases, specifically those acyltransferases transferring groups other than aminoacyl groups. The systematic name of this enzyme class is malonyl-CoA:cinnamoyl-CoA malonyltransferase (cyclizing). Other names in common use include stilbene synthase, and pine stilbene synthase. This enzyme participates in phenylpropanoid biosynthesis The biosynthesis of phenylpropanoids involves a number of enzymes. From amino acids to cinnamates In plants, all phenylpropanoids are derived from the amino acids phenylalanine and tyrosine. Phenylalanine ammonia-lyase (PAL, a.k.a. phenylalanine/t .... References * EC 2.3.1 Enzymes of unknown structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malonyl-CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid. Biosynthesis Malonyl-CoA cannot cross membranes and there is no known malonyl-CoA import mechanism. The biosynthesis therefore takes place locally: * cytosol: Malonyl-CoA is formed by carboxylating acetyl-CoA using the highly regulated enzyme acetyl-CoA carboxylase 1 (ACC1). One molecule of acetyl-CoA joins with a molecule of bicarbonate, requiring energy rendered from ATP. * Mitochondrial outer membrane: Malonyl-CoA is formed by carboxylating acetyl-CoA using the highly regulated enzyme acetyl-CoA carboxylase 2 (ACC2). The reaction is the same as with ACC1. * mitochondrial matrix: Malonyl-CoA is formed in coordinated fashion by mtACC1, a mitochondrial isoform of ACC1, and acyl-CoA synthetase family member 3 (ACSF3), a mitochondrial malonyl-CoA synthetase. MtACC1, like cytosolic ACC1 catalyses the carboxylation of acetyl-CoA, while ACSF3 catalyses the thioesterification of malonate to coenzyme A. The latter serves ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pinosylvin
Pinosylvin is an organic compound with the formula C6H5CH=CHC6H3(OH)2. A white solid, it is related to trans-stilbene, but with two hydroxy groups on one of the phenyl substituents. It is very soluble in many organic solvents, such as acetone. Occurrence Pinosylvin is produced in plants in response to fungal infections, ozone-induced stress, and physical damage for example. It is a fungitoxin protecting the wood from fungal infection. It is present in the heartwood of ''Pinaceae'' and also found in ''Gnetum cleistostachyum''. Injected in rats, pinosylvin undergoes rapid glucuronidation and a poor bioavailability. Biosynthesis Pinosylvin synthase, an enzyme, catalyzes the biosynthesis of pinosylvin from malonyl-CoA and cinnamoyl-CoA: :3 malonyl-S-CoA + cinnamoyl-S-CoA → 4 CoA-SH + pinosylvin + 4 CO2 This biosynthesis is noteworthy because plant biosyntheses employing cinnamic acid as a starting point are rare compared to the more common use of ''p''-coumaric acid. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioesters Of Coenzyme A
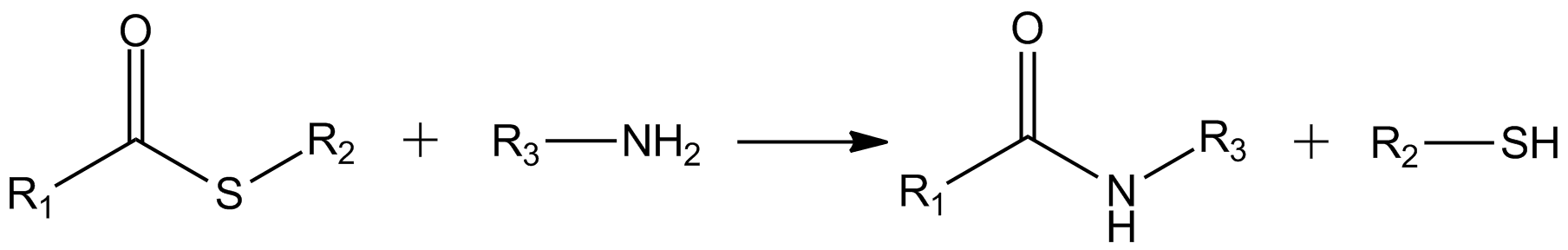
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid () with a thiol (). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. The R and R' represent organyl groups, or Hydrogen, H in the case of R. Synthesis One route to thioesters involves the reaction of an acid chloride with an alkali metal salt of a thiol: : Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate: : The a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |