|
Ciladopa
Ciladopa (AY-27,110) is a dopamine agonist with a similar chemical structure to dopamine. It was under investigation as an antiparkinsonian agent but was discontinued due to concerns of tumorogenesis Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnorm ... in rodents. References {{Phenethylamines Dopamine agonists Phenylethanolamines Piperazines Catechol ethers Tropones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
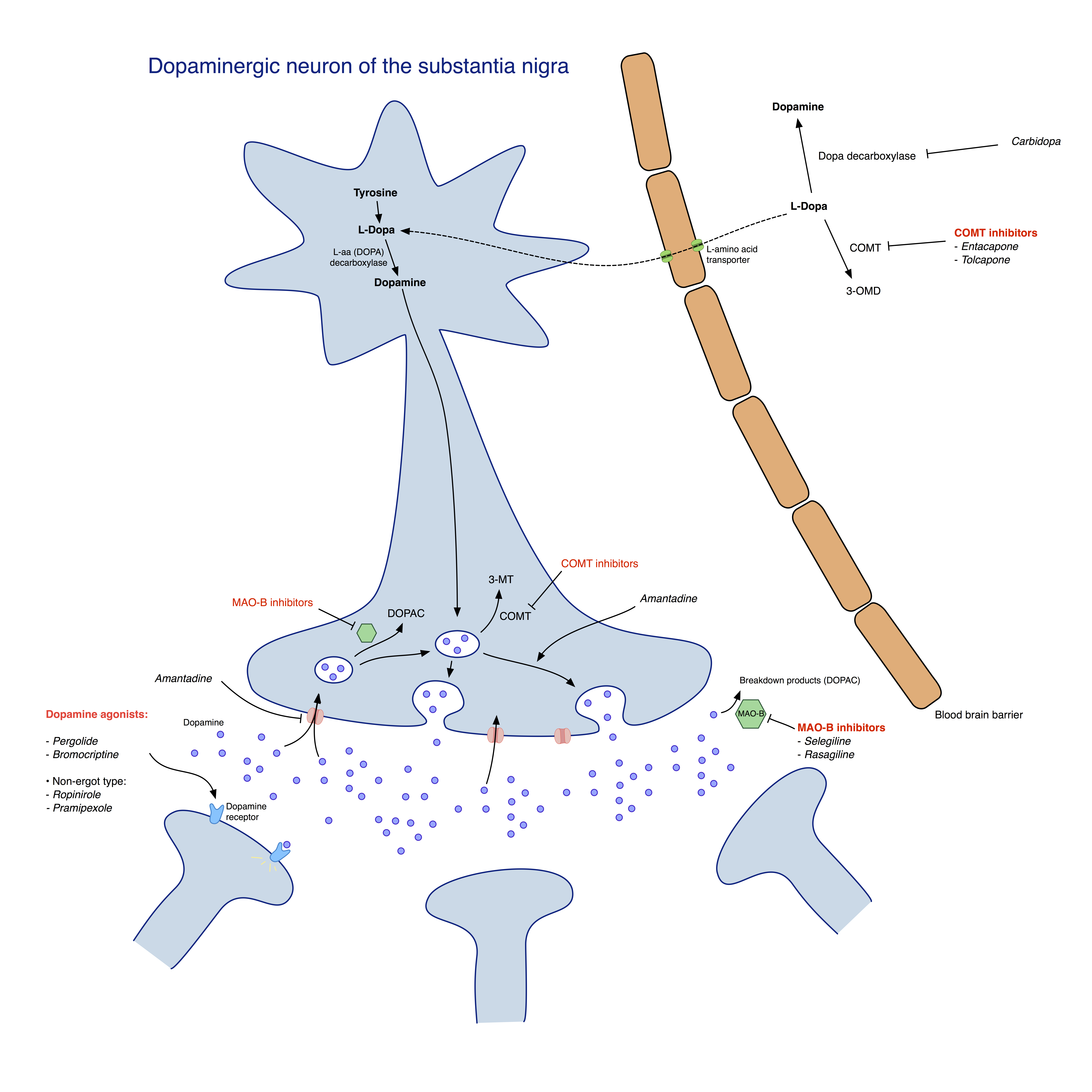
Dopamine Agonist
A dopamine agonist (DA) is a compound that activates dopamine receptors. There are two families of dopamine receptors, D2-like and D1-like, and they are all G protein-coupled receptors. D1- and D5-receptors belong to the D1-like family and the D2-like family includes D2, D3 and D4 receptors. Dopamine agonists are primarily used to treat Parkinson's disease. They are also used, to a far lesser extent, in treating hyperprolactinemia and restless legs syndrome. They are not intended for treatment of clinical depression, and studies have shown severe detrimental side effects resulting from off-label use of dopamine agonists in treating depression, particularly in their tendency to produce impulse control disorders and extreme cases of withdrawal syndrome. Medical uses Parkinson's disease Dopamine agonists are mainly used in the treatment of Parkinson's disease. The cause of Parkinson's is not fully known but genetic factors, for example specific genetic mutations, and enviro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine Agonists
A dopamine agonist (DA) is a compound that activates dopamine receptors. There are two families of dopamine receptors, D2-like and D1-like, and they are all G protein-coupled receptors. D1- and D5-receptors belong to the D1-like family and the D2-like family includes D2, D3 and D4 receptors. Dopamine agonists are primarily used to treat Parkinson's disease. They are also used, to a far lesser extent, in treating hyperprolactinemia and restless legs syndrome. They are not intended for treatment of clinical depression, and studies have shown severe detrimental side effects resulting from off-label use of dopamine agonists in treating depression, particularly in their tendency to produce impulse control disorders and extreme cases of withdrawal syndrome. Medical uses Parkinson's disease Dopamine agonists are mainly used in the treatment of Parkinson's disease. The cause of Parkinson's is not fully known but genetic factors, for example specific genetic mutations, and envi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
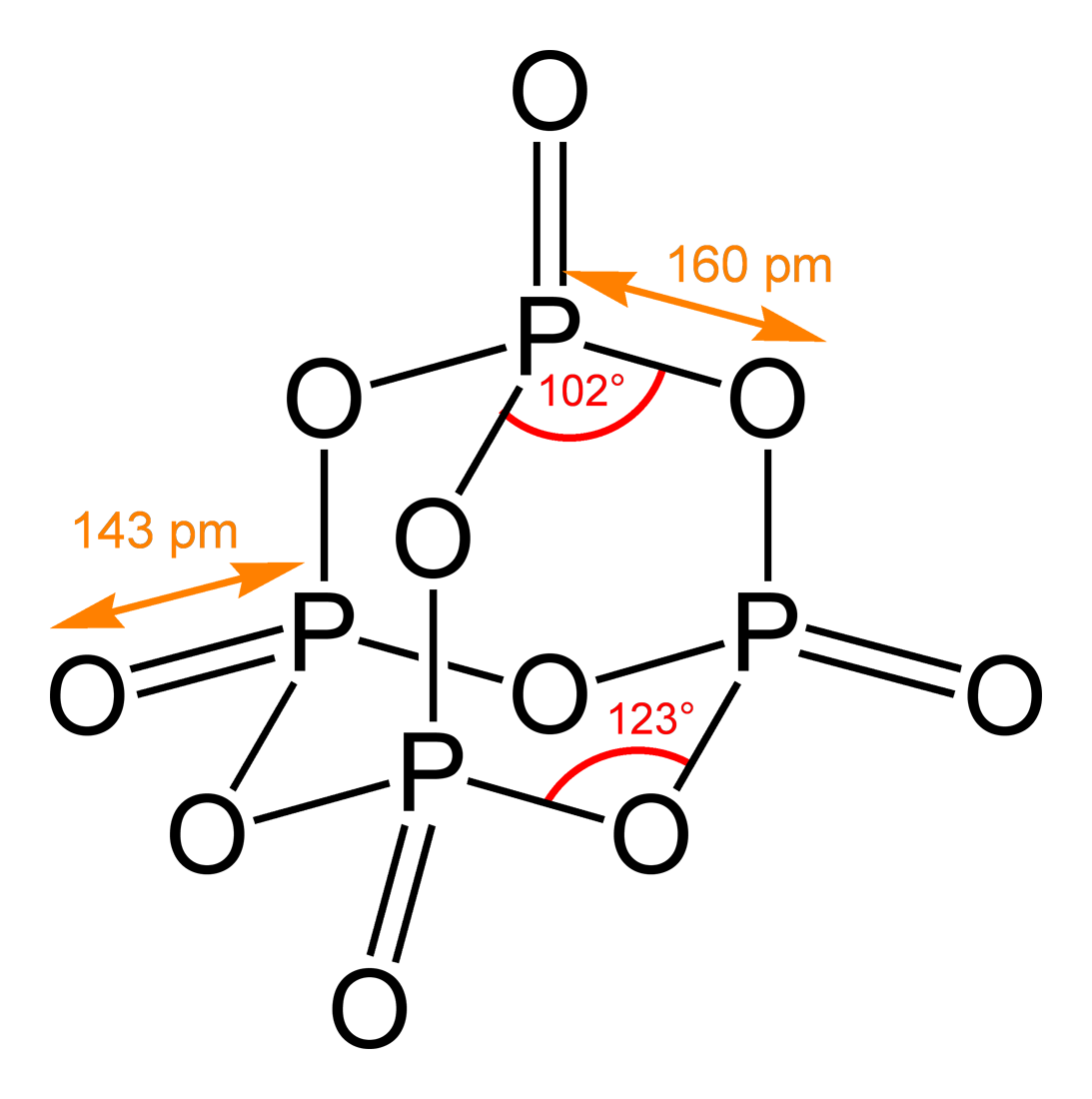
Chemical Structure
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together, and can be represented using structural formulae and by molecular models; complete electronic structure descriptions include specifying the occupation of a molecule's molecular orbitals. Structure determination can be applied to a range of targets from very simple molecules (e.g., diatomic oxygen or nitrogen), to very complex ones (e.g., such as protein or DNA). Background Theories of chemical structure were first developed by August Kekulé, Archibald Scott Couper, and Aleksandr Butlerov, among others, from about 1858. These theories were first to state that chemical compounds are not a random cluster of atoms and functional groups, but rather had a definite o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain, but affect many regions systemically. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Management Of Parkinson's Disease
In the management of Parkinson's disease, due to the chronic nature of Parkinson's disease (PD), a broad-based program is needed that includes patient and family education, support-group services, general wellness maintenance, exercise, and nutrition. At present, no cure for the disease is known, but medications or surgery can provide relief from the symptoms. While many medications treat Parkinson's, none actually reverses the effects of the disease. Furthermore, the gold-standard treatment varies with the disease state. People with Parkinson's, therefore, often must take a variety of medications to manage the disease's symptoms. Several medications currently in development seek to better address motor fluctuations and nonmotor symptoms of PD. However, none is yet on the market with specific approval to treat Parkinson's. Medication The main families of drugs useful for treating motor symptoms are levodopa, dopamine agonists, and MAO-B inhibitors. The most commonly used treatm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
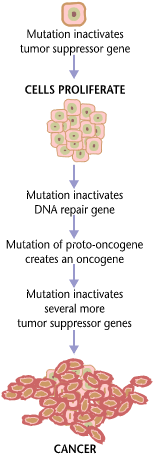
Tumorogenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnormal cell division. Cell division is a physiological process that occurs in almost all tissues and under a variety of circumstances. Normally, the balance between proliferation and programmed cell death, in the form of apoptosis, is maintained to ensure the integrity of tissues and organs. According to the prevailing accepted theory of carcinogenesis, the somatic mutation theory, mutations in DNA and epimutations that lead to cancer disrupt these orderly processes by interfering with the programming regulating the processes, upsetting the normal balance between proliferation and cell death. This results in uncontrolled cell division and the evolution of those cells by natural selection in the body. Only certain mutations lead to cancer w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperazines
Substituted piperazines are a class of chemical compounds based on a piperazine core. Some are used as recreational drugs and some are used in scientific research. List of substituted piperazines Benzylpiperazines File:Benzylpiperazine.svg, 1-Benzylpiperazine File:MBZP.svg, 1-Methyl-4-benzylpiperazine File:DBZP.svg, 1,4-Dibenzylpiperazine File:MDBZP.svg, 3,4-Methylenedioxy-1-benzylpiperazine File:2C-B-BZP.svg, 4-Bromo-2,5-dimethoxy-1-benzylpiperazine File:Methoxypiperamide.png, Methoxypiperamide File:Sunifiram.svg , Sunifiram File:3-Methylbenzylpiperazine structure.png, 3-Methylbenzylpiperazine * 1-Benzylpiperazine (BZP) * 1-Methyl-4-benzylpiperazine (MBZP) * 1,4-Dibenzylpiperazine (DBZP) * 3,4-Methylenedioxy-1-benzylpiperazine (MDBZP) * 4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP) * Methoxypiperamide (MeOP, MEXP) ((4-methoxyphenyl)(4-methylpiperazin-1-yl)methanone) * Sunifiram (1-benzoyl-4-propanoylpiperazine) * 3-Methylbenzylpiperazine (3-MeBZP) Befuralin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechol Ethers
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances. Catechol occurs as feathery white crystals that are very rapidly soluble in water. Isolation and synthesis Catechol was first isolated in 1839 by Edgar Hugo Emil Reinsch (1809–1884) by distilling it from the solid tannic preparation catechin, which is the residuum of catechu, the boiled or concentrated juice of ''Mimosa catechu'' ('' Acacia catechu''). Upon heating catechin above its decomposition point, a substance that Reinsch first named ''Brenz-Katechusäure'' (burned catechu aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |