|
Chromophore
A chromophore is the part of a molecule responsible for its color. The word is derived . The color that is seen by our eyes is that of the light not Absorption (electromagnetic radiation), absorbed by the reflecting object within a certain wavelength spectrum of visible spectrum, visible light. The chromophore is a region in the molecule where the energy difference between two separate molecular orbitals falls within the range of the visible spectrum (or in informal contexts, the spectrum under scrutiny). Visible light that hits the chromophore can thus be absorbed by exciting an electron from its ground state into an excited state. In biological molecules that serve to capture or detect light energy, the chromophore is the Moiety (chemistry), moiety that causes a conformational change in the molecule when hit by light. Conjugated pi-bond system chromophores Just like how two adjacent p-orbitals in a molecule will form a pi-bond, three or more adjacent p-orbitals in a molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision). Some microorganisms use retinal to convert light into metabolic energy. One study suggests that approximately three billion years ago, most living organisms on Earth used retinal, rather than chlorophyll, to convert sunlight into energy. Because retinal absorbs mostly green light and transmits purple light, this gave rise to the Purple Earth hypothesis. Retinal itself is considered to be a form of vitamin A when eaten by an animal. There are many forms of vitamin A, all of which are converted to retinal, which cannot be made without them. The number of different molecules that can be converted to retinal varies from species to species. Retinal was originally called retinene, and was renamed after it was discovered to be vitamin A aldehyde. Vertebrate animals inge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugated System
In physical organic chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases Chemical stability, stability. It is Resonance (chemistry), conventionally represented as having alternating single and multiple covalent bond, bonds. Lone pairs, radical (chemistry), radicals or carbenium ions may be part of the system, which may be Cyclic molecule, cyclic, acyclic, Linear molecular geometry, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele (chemist), Johannes Thiele. Conjugation is the orbital overlap, overlap of one p-orbital with another across an adjacent Sigma bond, σ bond (in transition metals, d-orbitals can be involved). A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond. They allow a delocalization of pi el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Visible Spectrum
The visible spectrum is the spectral band, band of the electromagnetic spectrum that is visual perception, visible to the human eye. Electromagnetic radiation in this range of wavelengths is called ''visible light'' (or simply light). The optical spectrum is sometimes considered to be the same as the visible spectrum, but some authors define the term more broadly, to include the ultraviolet and infrared parts of the electromagnetic spectrum as well, known collectively as ''optical radiation''. A typical human eye will respond to wavelengths from about 380 to about 750 nanometers. In terms of frequency, this corresponds to a band in the vicinity of 400–790 Terahertz (unit), terahertz. These boundaries are not sharply defined and may vary per individual. Under optimal conditions, these limits of human perception can extend to 310 nm (ultraviolet) and 1100 nm (near infrared). The spectrum does not contain all the colors that the human visual system can distinguish. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Electron Transition
In theoretical chemistry, molecular electronic transitions take place when electrons in a molecule are excited from one energy level to a higher energy level. The energy change associated with this transition provides information on the structure of the molecule and determines many of its properties, such as colour. The relationship between the energy involved in the electronic transition and the frequency of radiation is given by Planck's relation. Organic molecules and other molecules The electronic transitions in organic compounds and some other compounds can be determined by ultraviolet–visible spectroscopy, provided that transitions in the ultraviolet (UV) or visible range of the electromagnetic spectrum exist for the compound. Electrons occupying a HOMO (highest-occupied molecular orbital) of a sigma bond (σ) can get excited to the LUMO (lowest-unoccupied molecular orbital) of that bond. This process is denoted as a transition. Likewise, promotion of an electron fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conformational Change
In biochemistry, a conformational change is a change in the shape of a macromolecule, often induced by environmental factors. A macromolecule is usually flexible and dynamic. Its shape can change in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a ''conformational change''. Factors that may induce such changes include temperature, pH, voltage, light in chromophores, concentration of ions, phosphorylation, or the binding of a ligand. Transitions between these states occur on a variety of length scales (tenths of Å to nm) and time scales (ns to s), and have been linked to functionally relevant phenomena such as allosteric signaling and enzyme catalysis. Laboratory analysis Many biophysical techniques such as crystallography, NMR, electron paramagnetic resonance (EPR) using spin label techniques, circular dichroism (CD), hydrogen exchange, and FRET can be used to study macromo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fall Leaves (199582361)
Autumn, also known as fall (especially in US & Canada), is one of the four temperate seasons on Earth. Outside the tropics, autumn marks the transition from summer to winter, in September (Northern Hemisphere) or March ( Southern Hemisphere). Autumn is the season when the duration of daylight becomes noticeably shorter and the temperature cools considerably. Day length decreases and night length increases as the season progresses until the winter solstice in December (Northern Hemisphere) and June (Southern Hemisphere). One of its main features in temperate climates is the striking change in colour of the leaves of deciduous trees as they prepare to shed. Date definitions Some cultures regard the autumnal equinox as "mid-autumn", while others with a longer temperature lag treat the equinox as the start of autumn. In the English-speaking world of high latitude countries, autumn traditionally began with Lammas Day and ended around Hallowe'en, the approximate mid-points ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lycopene
Lycopene is an organic compound classified as a tetraterpene and a carotene. Lycopene (from the Neo-Latin '' Lycopersicon'', the name of a former tomato genus) is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables. Occurrence Aside from tomatoes or tomato products like ketchup, it is found in watermelons, grapefruits, red guavas, and baked beans. It has no vitamin A activity. In plants, algae, and other photosynthetic organisms, lycopene is an intermediate in the biosynthesis of many carotenoids, including beta-carotene, which is responsible for yellow, orange, or red pigmentation, photosynthesis, and photoprotection. Like all carotenoids, lycopene is a tetraterpene. It is soluble in fat, but insoluble in water. Eleven conjugated double bonds give lycopene its deep red color. Owing to the strong color, lycopene is used as a food coloring (registered as E160d) and is approved for use in the US, Australia and New Zealand (register ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PH Indicator
A pH indicator is a halochromism, halochromic chemical compound added in small amounts to a Solution (chemistry), solution so the pH (acidity or Base (chemistry), basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Hence, a pH indicator is a Chemical substance, chemical detector for hydronium ions (H3O+) or hydrogen ions (H+) in the Acid-base reaction theories, Arrhenius model. Normally, the indicator causes the color of the solution to change depending on the pH. Indicators can also show change in other physical properties; for example, olfactory indicators show change in their odor. The pH value of a neutral solution is 7.0 at 25°C (Standard conditions for temperature and pressure#Standard laboratory conditions, standard laboratory conditions). Solutions with a pH value below 7.0 are considered acidic and solutions with pH value above 7.0 are basic. Since most naturally occurring Organic compound, organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
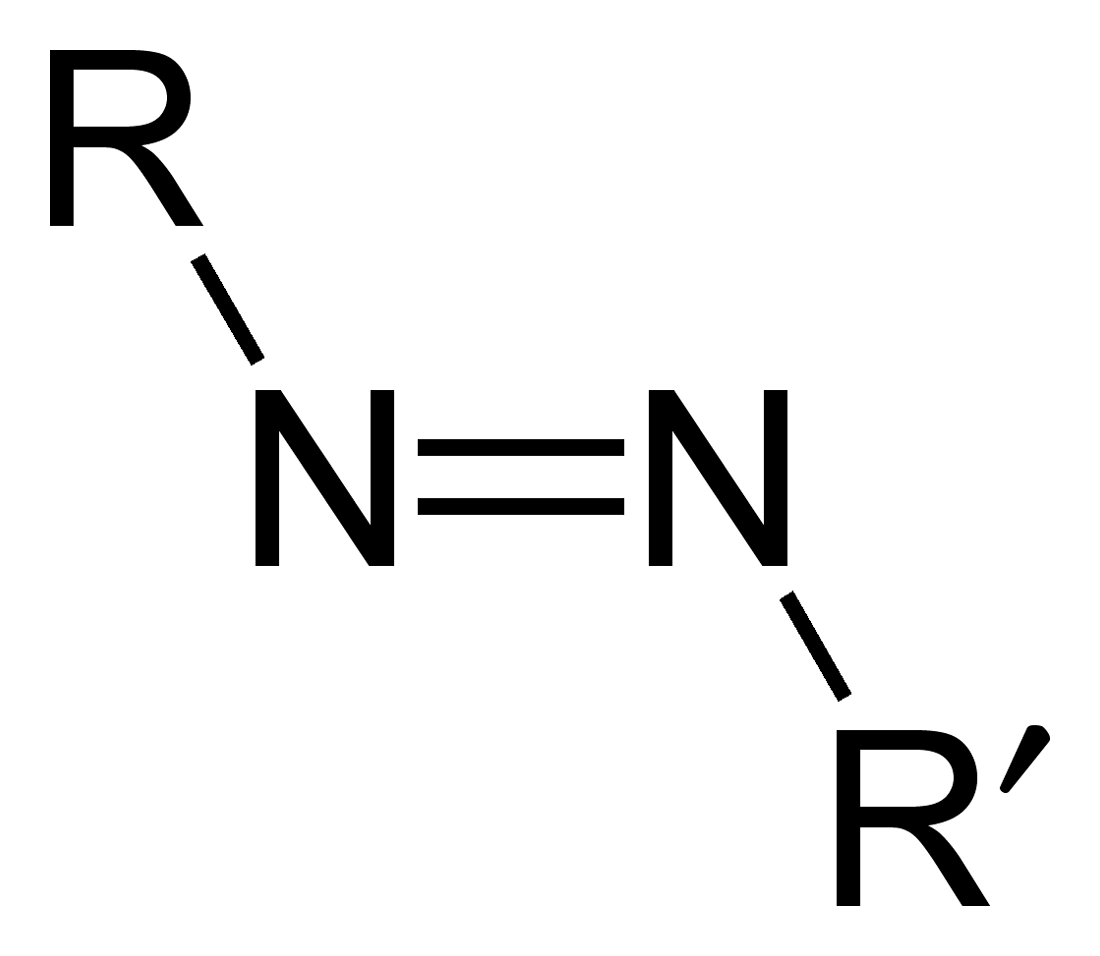
Azo Compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene.", where Ph stands for phenyl group. The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds urinary tract infections">Phenazopyridine, an aryl azo compound, is used to treat urinary tract infections">150px Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the Cis-trans isomerism, ''trans'' isomer, but upon illumination, converts to the Cis-trans isomerism, ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food Coloring
Food coloring, color additive or colorant is any dye, pigment, or substance that imparts color when it is added to food or beverages. Colorants can be supplied as liquids, powders, gels, or pastes. Food coloring is commonly used in commercial products and in domestic cooking. Food colorants are also used in various non-food applications, including cosmetics, pharmaceuticals, home craft projects, and medical devices. Some colorings may be natural, such as with carotenoids and anthocyanins extracted from plants or cochineal from insects, or may be synthesized, such as tartrazine yellow. In the manufacturing of foods, beverages and cosmetics, the safety of colorants is under constant scientific review and certification by national regulatory agencies, such as the European Food Safety Authority (EFSA) and US Food and Drug Administration (FDA), and by international reviewers, such as the Joint FAO/WHO Expert Committee on Food Additives. Purpose of food coloring People asso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |