 |
Chloromethane
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in industrial chemistry, although it is rarely present in consumer products, and was formerly utilized as a refrigerant. Most chloromethane is biogenic. Occurrence Chloromethane is an abundant organohalogen, anthropogenic or natural, in the atmosphere. Natural sources produce an estimated 4,100,000,000 kg/yr. Marine Laboratory cultures of marine phytoplankton (''Phaeodactylum tricornutum'', ''Phaeocystis'' sp., ''Thalassiosira weissflogii'', ''Chaetoceros calcitrans'', ''Isochrysis'' sp., ''Porphyridium'' sp., ''Synechococcus'' sp., ''Tetraselmis'' sp., ''Prorocentrum'' sp., and ''Emiliana huxleyi'') produce CH3Cl, but in relatively insignificant amounts. An extensive study of 30 species of polar macroalgae revealed the release of significant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Bromochloromethane
Bromochloromethane or methylene bromochloride and Halon 1011 is a mixed halomethane. It is a heavy low-viscosity liquid with refractive index 1.4808. Halon 1011 was invented for use in fire extinguishers in Germany during the mid-1940s, in an attempt to create a less toxic, more effective alternative to carbon tetrachloride. This was a concern in aircraft and tanks as carbon tetrachloride produced highly toxic by-products when discharged onto a fire. It was slightly less toxic, and used up until the late 1960s, being officially banned by the NFPA for use in fire extinguishers in 1969, as safer and more effective agents such as halon 1211 and 1301 were developed. Due to its ozone depletion potential its production was banned from January 1, 2002, at the ''Eleventh Meeting of the Parties for the Montreal Protocol on Substances that Deplete the Ozone Layer''. Bromochloromethane's biodegradation is catalyzed by the hydrolase enzyme alkylhalidase: :CH2BrCl + H2O → CH2O + HBr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Dibromochloromethane
Dibromochloromethane is a colorless to yellow, heavy and nonflammable compound with formula . It is a trihalomethane. The substance has a sweet odour. Small quantities of dibromochloromethane are produced in ocean by algae. Applications Dibromochloromethane was formerly used as a flame retardant and as an intermediate in chemicals manufacturing. Today it is used only as a laboratory reagent. Dibromochloromethane is also a disinfection byproduct, formed by the reaction of chlorine with natural organic matter and bromide ions in the raw water supply. As a result, it is commonly found in chlorinated drinking water. Also, it is able to reduce methane production in ruminants by 79 % See also * Asparagopsis taxiformis References External links Dibromochlormethane in greenfacts.org glossaryToxFAQ for bromoform at ATSDR {{halomethanes Bromochloroalkanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Haloalkane
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguisher, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Organochlorine Chemistry
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds that contain one or more carbon–chlorine bonds. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Organochlorides such as trichloroethylene, tetrachloroethylene, dichloromethane and chloroform are commonly used as solvents and are referred to as "chlorinated solvents". Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. They have higher ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Chloroiodomethane
Chloroiodomethane is the halomethane with the formula is . It is a colorless liquid of use in organic synthesis. Together with other iodomethanes, chloroiodomethane is produced by some microorganisms. Applications Chloroiodomethane is used in cyclopropanation (''Simmon-Smith reaction''), where it often replaces diiodomethane because of higher yields and selectivity. It is also used in Mannich reaction, aminomethylation, epoxidation, ring opening and addition to terminal alkenes. It is a precursor to agent Ph3P=CHCl that can add a chloromethylene group (=CHCl). It reacts with organolithium compounds to give chloromethyl lithium (ClCH2Li). Crystallography It crystallizes orthorhombic crystal system In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with ... with space group ''Pnma'' wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
2-Chloroethanol
2-Chloroethanol (also called ethylene chlorohydrin or glycol chlorohydrin) is an organic chemical compound with the chemical formula HOCH2CH2Cl and the ''simplest'' beta-halohydrin (chlorohydrin). This colorless liquid has a pleasant ether-like odor. It is miscible with water. The molecule is bifunctional, consisting of both an alkyl chloride and an alcohol functional group. Synthesis and applications 2-Chloroethanol is produced by treating ethylene with hypochlorous acid: : 2-Chloroethanol was once produced on a large scale as a precursor to ethylene oxide: : :HOCH2CH2Cl + NaOH → C2H4O + NaCl + H2O This application has been supplanted by the more economic direct oxidation of ethylene. Otherwise chloroethanol is still used in the production of pharmaceuticals, biocides, and plasticizers. Many of these applications entail its use in installing 2-hydroxyethyl groups. Several dyes are prepared by the alkylation of aniline derivatives with chloroethanol. It is also used for manuf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Methyl Chloride Transferase
Methyl halide transferase (, ''MCT'', methyl chloride transferase, ''S-adenosyl-L-methionine:halide/bisulfide methyltransferase'', ''AtHOL1'', ''AtHOL2'', ''AtHOL3'', ''HMT'', ''S-adenosyl-L-methionine: halide ion methyltransferase'', ''SAM:halide ion methyltransferase'') is an enzyme with systematic name ''S-adenosylmethionine:iodide methyltransferase''. This enzyme catalyses the following chemical reaction : ''S''-adenosyl-L-methionine + iodide \rightleftharpoons ''S''-adenosyl-L-homocysteine + methyl iodide This enzyme contributes to the methyl halide emissions from ''Arabidopsis thaliana''. Chloride transfer The salt marsh plant ''Batis maritima'' contains the enzyme methyl chloride transferase that catalyzes the synthesis of chloromethane (CH3Cl) from S-adenosine-L-methionine and chloride. This protein has been purified and expressed in ''E. coli'', and seems to be present in other organisms such as white rot fungi (''Phellinus pomaceus''), red algae ('' Endocladia muric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Organohalogen
Halocarbon compounds are chemical compounds in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – ) resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds. Chlorine halocarbons are the most common and are called organochlorides. Many synthetic organic compounds such as plastic polymers, and a few natural ones, contain halogen atoms; they are known as ''halogenated'' compounds or ''organohalogens''. Organochlorides are the most common industrially used organohalides, although the other organohalides are used commonly in organic synthesis. Except for extremely rare cases, organohalides are not produced biologically, but many pharmaceuticals are organohalides. Notably, many pharmaceuticals such as Prozac have trifluoromethyl groups. For information on inorganic halide chemistry, see halide. Chemical families Halocar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Star Forming Region
A star is a luminous spheroid of plasma held together by self-gravity. The nearest star to Earth is the Sun. Many other stars are visible to the naked eye at night; their immense distances from Earth make them appear as fixed points of light. The most prominent stars have been categorised into constellations and asterisms, and many of the brightest stars have proper names. Astronomers have assembled star catalogues that identify the known stars and provide standardized stellar designations. The observable universe contains an estimated to stars. Only about 4,000 of these stars are visible to the naked eye—all within the Milky Way galaxy. A star's life begins with the gravitational collapse of a gaseous nebula of material largely comprising hydrogen, helium, and traces of heavier elements. Its total mass mainly determines its evolution and eventual fate. A star shines for most of its active life due to the thermonuclear fusion of hydrogen into helium in its core. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
67P/Churyumov–Gerasimenko
67P/Churyumov–Gerasimenko (abbreviated as 67P or 67P/C–G) is a Jupiter-family comet. It is originally from the Kuiper belt and has an orbital period of 6.45 years as of 2012, a rotation period of approximately 12.4 hours, and a maximum velocity of . Churyumov–Gerasimenko is approximately at its longest and widest dimensions. It was first observed on photographic plates in 1969 by Soviet astronomers Klim Churyumov, Klim Ivanovych Churyumov and Svetlana Gerasimenko, Svetlana Ivanovna Gerasimenko, after whom it is Naming of comets, named. It most recently came to Perihelion and aphelion, perihelion (closest approach to the Sun) on 2 November 2021, and will next come to perihelion on 9 April 2028. Churyumov–Gerasimenko was the destination of the European Space Agency's Rosetta (spacecraft), ''Rosetta'' mission, launched on 2 March 2004. ''Rosetta'' space rendezvous, rendezvoused with Churyumov–Gerasimenko on 6 August 2014 and entered orbit on 10 September 2014. ''Rosetta' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
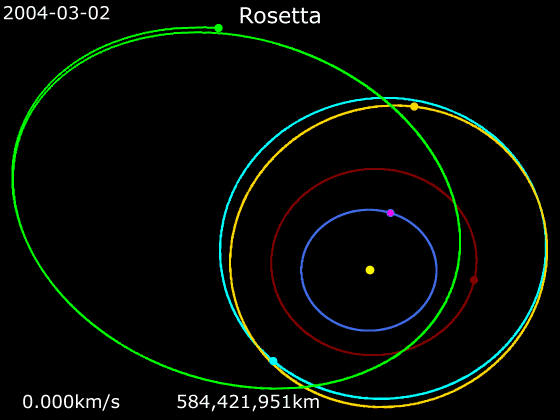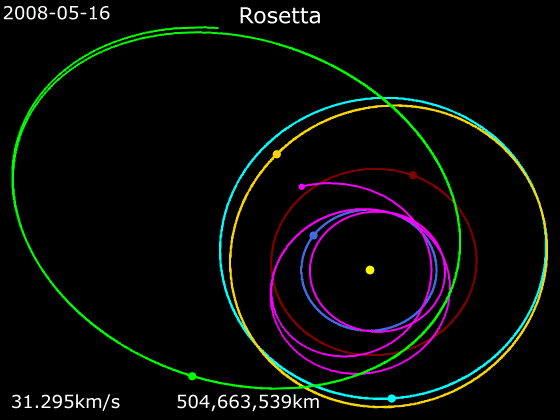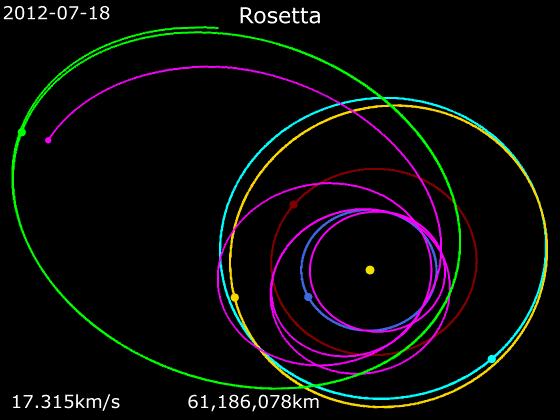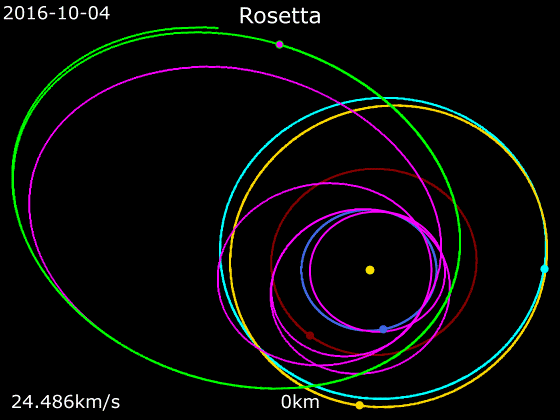 |
Rosetta (spacecraft)
''Rosetta'' was a space probe built by the European Space Agency that launched on 2 March 2004. Along with ''Philae'', its lander module, ''Rosetta'' performed a detailed study of comet 67P/Churyumov–Gerasimenko (67P). During its journey to the comet, the spacecraft performed flybys of Earth, Mars, and the asteroids 21 Lutetia and 2867 Šteins. It was launched as the third cornerstone mission of the ESA's Horizon 2000 programme, after ''SOHO' Cluster'' and '' XMM-Newton''. On 6 August 2014, the spacecraft reached the comet and performed a series of manoeuvers to eventually orbit the comet at distances of . On 12 November, its lander module ''Philae'' performed the first successful landing on a comet, though its battery power ran out two days later. Communications with ''Philae'' were briefly restored in June and July 2015, but due to diminishing solar power, ''Rosetta'' communications module with the lander was turned off on 27 July 2016. On 30 September 2016, the ''Rosett ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |