|
Chlorethoxyfos
Chlorethoxyfos (''O'',''O''-diethyl-''O''-(1,2,2,2-tetrachloroethyl)phosphorothioate) is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is registered for the control of corn rootworms, wireworms, cutworms, seed corn maggot, white grubs and symphylans on corn. The insecticide is sold under the trade name Fortress by E.I. du Pont de Nemours & Company. Annual domestic usage of chlorethoxyfos is estimated to range from 8,500 to 17,800 pounds of active ingredient for approximately 37,000 to 122,000 acres treated. Approximately 1% of all corn acreage is treated. Chlorethoxyfos has a ''O''-alkyl phosphorothioate type of phosphorus group which makes it similar to compounds such as chlorpyriphos-methyl, coumaphos, diazinon, dichlofenthion, fenitrothion, fenthion, parathion, parathion-methyl, pyrazophos, pyrimiphos-methyl, sulfotep, temephos, and thionazin. The compound does not have EU regulatory approval for use as an insecticide as it can be harmf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to be a major factor behind the increase in the 20th-century's agricultural productivity. Nearly all insecticides have the potential to significantly alter ecosystems; many are toxic to humans and/or animals; some become concentrated as they spread along the food chain. Insecticides can be classified into two major groups: systemic insecticides, which have residual or long term activity; and contact insecticides, which have no residual activity. The mode of action describes how the pesticide kills or inactivates a pest. It provides another way of classifying insecticides. Mode of action can be important in understanding whether an insecticide will be toxic to unrelated species, such as fish, birds and mammals. Insecticides may be repe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphate
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Like most functional groups, organophosphates occur in a diverse range of forms, with important examples including key biomolecules such as DNA, RNA and ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The popularity of OPEs as flame retardants came as a substitution for the highly regulated brominated flame retardants. The low cost of production and compatibility to diverse polymers made OPEs to be widely used in industry including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added to the final product phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pirimiphos-methyl
Pirimiphos-methyl, marketed as Actellic, and Sybol is a phosphorothioate used as an insecticide. It was originally developed by Imperial Chemical Industries Ltd., now Syngenta, at their Jealott's Hill Jealott's Hill is a village in the county of Berkshire, England, within the civil parish of Warfield. The settlement is on the A3095 road approximately north of Bracknell. The nearest railway station is in . The name of the hill is reported to ... site and first marketed in 1977, ten years after its discovery. This is one of several compounds used for vector control of '' Triatoma''. These insects are implicated in the transmission of Chagas disease in the Americas. Vectors of Chagas disease, World Health Organization Pirimiphos-methyl can be applied as an interior surface p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Pentachloride
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride. Structure The structures for the phosphorus chlorides are invariably consistent with VSEPR theory. The structure of PCl5 depends on its environment. Gaseous and molten PCl5 is a neutral molecule with trigonal bipyramidal geometry and (''D''3h) symmetry. The hypervalent nature of this species (as well as of , see below) can be explained with the inclusion of non-bonding molecular orbitals (molecular orbital theory) or resonance (valence bond theory). This trigonal bipyramidal structure persists in nonpolar solvents, such as CS2 and CCl4. In the solid state PCl5 is an ionic compound, formulated . In solutions of polar solvents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichloroacetic Acid
Trichloroacetic acid (TCA; TCAA; also known as trichloroethanoic acid) is an analogue of acetic acid in which the three hydrogen atoms of the methyl group have all been replaced by chlorine atoms. Salts and esters of trichloroacetic acid are called trichloroacetates. Synthesis It is prepared by the reaction of chlorine with acetic acid in the presence of a suitable catalyst such as red phosphorus. This reaction is Hell–Volhard–Zelinsky halogenation. : + 3 → + 3 Another route to trichloroacetic acid is the oxidation of trichloroacetaldehyde. Use It is widely used in biochemistry for the precipitation of macromolecules, such as proteins, DNA, and RNA. TCA and DCA are both used in cosmetic treatments (such as chemical peels and tattoo removal) and as topical medication for chemoablation of warts, including genital warts. It can kill normal cells as well. It is considered safe for use for this purpose during pregnancy. The sodium salt ( sodium trichloroacetat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichloroacetic Acid
Dichloroacetic acid (DCA), sometimes called bichloroacetic acid (BCA), is the chemical compound with formula . It is an acid, an analogue of acetic acid, in which 2 of the 3 hydrogen atoms of the methyl group have been replaced by chlorine atoms. Like the other chloroacetic acids, it has various practical applications. The salts and esters of dichloroacetic acid are called dichloroacetates. Salts of DCA have been studied as potential drugs because they inhibit the enzyme pyruvate dehydrogenase kinase. Although preliminary studies found that DCA can slow the growth of certain tumors in animal studies and ''in vitro'' studies, as of 2012 insufficient evidence supported the use of DCA for cancer treatment. Chemistry and occurrence The chemistry of dichloroacetic acid is typical for halogenated organic acids. It is a member of the chloroacetic acids family. The dichloroacetate ion is produced when the acid is mixed with water. As an acid with a p''K''a of 1.35, pure dichloroac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esterase
An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis. A wide range of different esterases exist that differ in their substrate specificity, their protein structure, and their biological function. EC classification/list of enzymes * ''EC 3.1.1'': Carboxylic ester hydrolases ** Acetylesterase (EC 3.1.1.6), splits off acetyl groups *** Cholinesterase **** Acetylcholinesterase, inactivates the neurotransmitter acetylcholine **** Pseudocholinesterase, broad substrate specificity, found in the blood plasma and in the liver ** Pectinesterase (EC 3.1.1.11), clarifies fruit juices * ''EC 3.1.2'': Thiolester hydrolases ** Thioesterase *** Ubiquitin carboxy-terminal hydrolase L1 * ''EC 3.1.3'': Phosphoric monoester hydrolases ** Phosphatase (EC 3.1.3.x), hydrolyses phosphoric acid monoesters into a phosphate ion and an alcohol *** Alkaline phosphatase, removes phosphate groups from many types ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desulfurization
Desulfurization or desulphurisation is a chemical process for the removal of sulfur from a material. This involves either the removal of sulfur from a molecule (''e.g.'' A=S → A:) or the removal of sulfur compounds from a mixture such as oil refinery streams. These processes are of great industrial and environmental importance as they provide the bulk of sulfur used in industry (Claus process and Contact process), sulfur-free compounds that could otherwise not be used in a great number of catalytic processes, and also reduce the release of harmful sulfur compounds into the environment, particularly sulfur dioxide (SO2) which leads to acid rain. Processes used for desulfurization include hydrodesulfurization, SNOX process and the wet sulfuric acid process (WSA process). See also * Shell–Paques process * Flue-gas desulfurization * Biodesulfurization Biodesulfurization is the process of removing sulfur from crude oil through the use of microorganisms or their enzymes. Backgr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
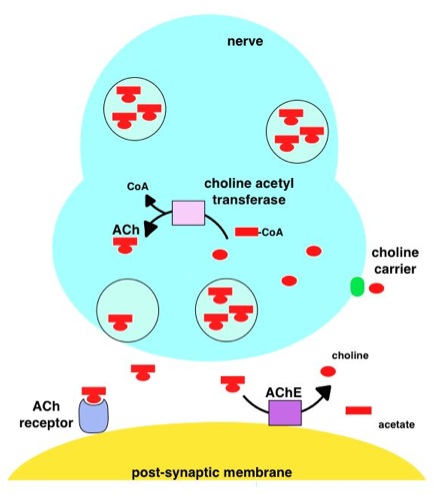
Acetylcholine
Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic. Substances that increase or decrease the overall activity of the cholinergic system are called cholinergics and anticholinergics, respectively. Acetylcholine is the neurotransmitter used at the neuromuscular junction—in other words, it is the chemical that motor neurons of the nervous system release in order to activate muscles. This property means that drugs that affect cholinergic systems can have very dangerous effects ranging from paralysis to convulsions. Acetylcholine is also a neurotransmitter in the autonomic nervous system, both as an internal transmitter for the sympathetic nervous system and as the final product released by the para ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters: : acetylcholine + H2O = choline + acetate It is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. Enzyme structure and mechanism AChE is a hydrolase that hydrolyzes choline esters. It has a very high catalytic activity—each molecule of AChE degrades about 25,000 molecules of acetylcholine (ACh) per second, approaching the limit allowed by diffusion of the substrate. The active site of AChE comprises 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |