|
Chemical Drain Cleaner
Chemical drain cleaners or openers are pure or mixtures of chemicals used to unclog drains that are blocked by hair, food, or other organic materials. They are often accompanied by other mechanical drain cleaners for the optimal effect. Chemical drain cleaners are available through hardware stores, although some may be intended for use by licensed plumbers. They may contain either strong acids (in liquid forms) or strong alkalis (in either solid or liquid forms). These cleaners contain chemicals that dissolve at least some of the material causing the clog. History The history of drain cleaners parallels the development of common drain systems themselves. As a result, there is not an extensive history of cleaners in the US, as municipal plumbing systems were not readily available in middle-class American homes until the early 20th century. Prior to this time, Americans often discarded the dirty water collected in basins after use. Limited piping systems gradually developed with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drain Cleaners
A drain cleaner, also known as drain opener, refers to a person, device, or product used to unblock sewer pipes or clear clogged wastewater drains. This term typically applies to chemical, enzymatic, or mechanical tools such as commercial chemical cleaners, plumber's snakes, drain augers, bio-enzyme solutions, or toilet plungers. In some contexts, it may also refer to a plumber or professional who specializes in drain cleaning and maintenance. Chemical drain cleaners, plungers, handheld drain augers, and air burst drain cleaners are typically used to address clogs in single drain, such as sinks, toilets, tubs, or shower drains. These tools are effective at removing soft obstructions like hair and grease that accumulate near the drain inlet. However, excessive use of chemical drain cleaners can lead to pipe damage. In contrast, enzymatic drain cleaners rely on natural enzymes to break down organic matter such as grease, hair, and food particles, offering a more environmentally fri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the cation , also written as , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton (a positive hydrogen ion, ) to the surrounding water molecules (). In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous and conjugate base. Three main structures for the aqueous proton have garnered experimental support: * the Eigen cation, which is a tetrahydrate, H3O+(H2O)3 * the Zundel cation, which is a symmetric dihydrate, H+(H2O)2 * and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H+(H2O)2(H2O)4 Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form. For this reason, it has been suggested that wherever possible, the sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
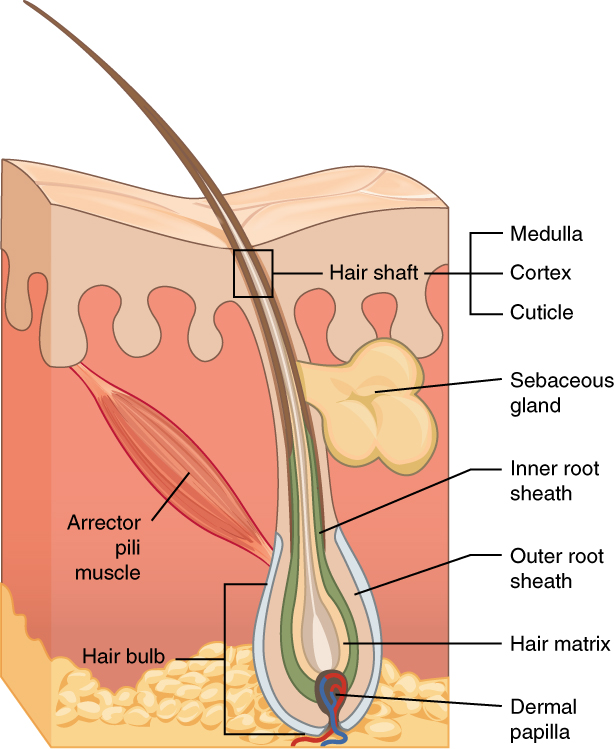
Hair
Hair is a protein filament that grows from follicles found in the dermis. Hair is one of the defining characteristics of mammals. The human body, apart from areas of glabrous skin, is covered in follicles which produce thick terminal and fine vellus hair. Most common interest in hair is focused on hair growth, hair types, and hair care, but hair is also an important biomaterial primarily composed of protein, notably alpha-keratin. Attitudes towards different forms of hair, such as hairstyles and hair removal, vary widely across different cultures and historical periods, but it is often used to indicate a person's personal beliefs or social position, such as their age, gender, or religion. Overview Meaning The word "hair" usually refers to two distinct structures: #the part beneath the skin, called the hair follicle, or, when pulled from the skin, the bulb or root. This organ is located in the dermis and maintains stem cells, which not only re-grow the hair afte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol () is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is Hygroscopy, hygroscopic in nature. Modern use of the word glycerine (alternatively spelled glycerin) refers to commercial preparations of less than 100% purity, typically 95% glycerol. Structure Although chirality, achiral, glycerol is prochirality, prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the Glycerophospholipid#Nomenclature and stereochemistry, stereospecific numbering labels the molecule with a ''sn''- prefix before the stem name of the molecule. Production Natural sources Glycerol is generally obtained from plant and animal sources where it occurs in triglycerides, est ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyceryl Trioleate
Triolein (glyceryl trioleate) is a symmetrical triglyceride derived from glycerol and three units of the unsaturated fatty acid oleic acid. Most triglycerides are unsymmetrical, being derived from mixtures of fatty acids. Triolein represents 4–30% of olive oil. Triolein is also known as glyceryl trioleate and is one of the two components of Lorenzo's oil. The oxidation of triolein is according to the formula: : + 80 → 57 + 52 This gives a respiratory quotient The respiratory quotient (RQ or respiratory coefficient) is a dimensionless number used in calculations of basal metabolic rate (BMR) when estimated from carbon dioxide production. It is calculated from the ratio of carbon dioxide produced by the ... of 57/80 or 0.7125. The heat of combustion is per mole or per gram. Per mole of oxygen it is . References Triglycerides {{ester-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saponification
Saponification is a process of cleaving esters into carboxylate salts and Alcohol (chemistry), alcohols by the action of aqueous alkali. Typically aqueous sodium hydroxide solutions are used. It is an important type of alkaline hydrolysis. When the carboxylate is long chain, its salt is called a soap. The saponification of ethyl acetate gives sodium acetate and ethanol: : Saponification of fats Vegetable oils and animal fats are the traditional materials that are saponified. These greasy materials, triesters called triglycerides, are usually mixtures derived from diverse fatty acids. In the traditional saponification, the triglyceride is treated with lye, which cleaves the ester bonds, releasing fatty acid salts (soaps) and glycerol. In one simplified version, the saponification of stearin gives sodium stearate. : This process is the main industrial method for producing glycerol (). Some soap-makers leave the glycerol in the soap. Others precipitation (chemistry), precipitate t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash. Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utilize its caustic nature and its reactivity toward acids. An estimated 700,000 to 800,000 tonnes were produced in 2005. KOH is noteworthy as the precursor to most soft and liquid soaps, as well as numerous potassium-containing chemicals. It is a white solid that is dangerously corrosive. Properties and structure KOH exhibits high thermal stability. Because of this high stability and relatively low melting point, it is often melt-cast as pellets or rods, forms that have low surface area and convenient handling properties. These pellets become tacky in air because KOH is hygroscopic. Most commercial samples are ca. 90% pure, the remainder being water and carbonates. Its dissolution in water is strongly exothermic. Concentrated aqueous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly corrosive base (chemistry), base and alkali that decomposes lipids and proteins at ambient temperatures and at high concentrations may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the making of wood pulp and paper, tex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most abundant biopolymer, organic polymer on Earth. The cellulose content of cotton fibre is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%. Cellulose is mainly used to produce paperboard and paper. Smaller quantities are converted into a wide variety of derivative products such as cellophane and rayon. Conversion of cellulose from energy crops into biofuels such as cellulosic ethanol is under development as a renewable fuel source. Cellulose for industrial use is mainly obtained from wood pulp and cotton. Cellulose is also greatly affected by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tissue Paper
Tissue paper, or simply tissue, is a lightweight paper or light crêpe paper. Tissue can be made from recycled pulp (paper), paper pulp on a paper machine. Tissue paper is very versatile, and different kinds are made to best serve these purposes, which are hygienic tissue paper, facial tissues, paper towels, as packing material, among other (sometimes creative) uses. The use of tissue paper is common in developed nations, around 21 million tonnes in North America and 6 million in Europe, and is growing due to urbanization. As a result, the industry has often been scrutinized for deforestation. However, more companies are presently using more recycled fibres in tissue paper. Properties The key properties of tissues are absorbency, basis weight, thickness, bulk (specific volume), brightness, stretch, appearance and comfort. Production Tissue paper is produced on a Fourdrinier machine, paper machine that has a single large steam heated drying cylinder (Yankee dryer) fitted with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |