|
Cericlamine
Cericlamine (INN; developmental code JO-1017) is a potent and moderately selective serotonin reuptake inhibitor (SSRI) of the amphetamine family (specifically, a derivative of phentermine, and closely related to chlorphentermine, a highly selective serotonin releasing agent) that was investigated as an antidepressant for the treatment of depression, anxiety disorders, and anorexia nervosa by Jouveinal but did not complete development and was never marketed. It reached phase III clinical trials in 1996 before development was discontinued in 1999. Synthesis Arylation of methacrylic acid with a diazonium salt of 3,4-dichloroaniline (or 3,4-dichloro-benzenediazonium salt), is carried out according to the Meerwein reaction catalysed by a metallic halide. For the next step, the halide is displaced by dimethylamine, then esterification is performed, followed by reduction with a metal hydride. See also * 3,4-Dichloroamphetamine * Alaproclate * Bupropion * Chlorphentermine * Cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cericlamine Synthesis
Cericlamine (INN; developmental code JO-1017) is a potent and moderately selective serotonin reuptake inhibitor (SSRI) of the amphetamine family (specifically, a derivative of phentermine, and closely related to chlorphentermine, a highly selective serotonin releasing agent) that was investigated as an antidepressant for the treatment of depression, anxiety disorders, and anorexia nervosa by Jouveinal but did not complete development and was never marketed. It reached phase III clinical trials in 1996 before development was discontinued in 1999. Synthesis Arylation of methacrylic acid with a diazonium salt of 3,4-dichloroaniline (or 3,4-dichloro-benzenediazonium salt), is carried out according to the Meerwein reaction catalysed by a metallic halide. For the next step, the halide is displaced by dimethylamine, then esterification is performed, followed by reduction with a metal hydride. See also * 3,4-Dichloroamphetamine * Alaproclate * Bupropion * Chlorphentermine * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selective Serotonin Reuptake Inhibitor
Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions. SSRIs increase the extracellular level of the neurotransmitter serotonin by Reuptake inhibitor, limiting its reuptake, reabsorption (reuptake) into the presynaptic cell. They have varying degrees of selectivity for the other monoamine transporters, with pure SSRIs having strong affinity for the serotonin transporter and only weak affinity for the norepinephrine transporter, norepinephrine and dopamine transporters. SSRIs are the most widely prescribed antidepressants in many countries. The efficacy of SSRIs in mild or moderate cases of depression has been disputed and may or may not be outweighed by side effects, especially in adolescent populations. Medical uses The main indication for SSRIs is major depressive disorder; however, they are frequently prescribed for a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3,4-Dichloroamphetamine
3,4-Dichloroamphetamine (DCA), is an amphetamine derived drug invented by Eli Lilly in the 1960s, which has a number of pharmacological actions. It acts as a highly potent and selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with high affinity, but also acts as a selective serotonergic neurotoxin in a similar manner to the related para-chloroamphetamine, though with slightly lower potency. It is also a monoamine oxidase inhibitor (MAOI), as well as a very potent inhibitor of the enzyme phenylethanolamine N-methyl transferase which normally functions to transform noradrenaline into adrenaline in the body. Synthesis The reaction of 3,4-Dichlorobenzyl Chloride 02-47-6(1) with cyanide anion gives 3,4-Dichlorophenylacetonitrile 218-49-3(2). Reaction with sodium methoxide and ethylacetate gives Alpha-Acetoxy-3,4-DichlorobenzeneacetonitrileCID:14318103(3). Removal of the nitrile group in the presence of sulfuric acid gives 3,4-Dichlorophenylacet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clortermine
Clortermine (Voranil) was developed by Ciba in the 1960s and is an anorectic drug of the amphetamine class. It is the 2-chloro analogue of the better known appetite suppressant phentermine, and is the 2-chloro positional isomer of chlorphentermine. Clortermine produces very low rates of self-administration in animals similarly to chlorphentermine, and as a result it likely does not act on dopamine. Instead, it may act as a serotonin and/or norepinephrine releasing agent. See also * 3,4-Dichloroamphetamine * Cericlamine * Chlorphentermine * Cloforex * Etolorex * Methylenedioxyphentermine * Phentermine Phentermine ( phenyl- tertiary-butyl amine), with several brand names including Ionamin and Sentis, is a medication used together with diet and exercise to treat obesity. It is taken by mouth for up to a few weeks at a time, after which the ben ... References Anorectics Chloroarenes Monoamine releasing agents Substituted amphetamines {{Nervous-system-drug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylenedioxyphentermine
3,4-Methylenedioxyphentermine (MDPH) is a lesser-known psychedelic drug. MDPH was first synthesized by Alexander Shulgin. In his book '' PiHKAL (Phenethylamines i Have Known And Loved)'', the dosage range is listed as 160–240 mg, and the duration as 3–5 hours. MDPH's effects are very similar to those of MDA: they both are smooth and "stoning," and do not cause any visuals. They also alter dreams and dream patterns. Shulgin describes MDPH as a promoter; it promotes the effects of other drugs, similarly to 2C-D. Very little data exists about the pharmacological properties, metabolism, and toxicity of MDPH. Legality United Kingdom This substance is a Class A drug in the Drugs controlled by the UK Misuse of Drugs Act. See also * 3,4-Dichloroamphetamine * Cericlamine * Chlorphentermine * Cloforex * Clortermine * Etolorex * Phentermine Phentermine ( phenyl- tertiary-butyl amine), with several brand names including Ionamin and Sentis, is a medication used together w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorphentermine
Chlorphentermine (trade names Apsedon, Desopimon, Lucofen) is a serotonergic appetite suppressant of the amphetamine family. Developed in 1962, it is the 4-chloro derivative of the better known appetite suppressant phentermine, which is still in current use. Chlorphentermine acts as a highly selective serotonin releasing agent (SRA). It is not a psychostimulant and has little or no abuse potential, but is classed as a Schedule III drug in the USA due mainly to its similarity to other appetite suppressants such as diethylpropion which have been more widely abused. It is no longer used due mainly to safety concerns, as it has a serotonergic effects profile similar to other withdrawn appetite suppressants such as fenfluramine and aminorex which were found to cause pulmonary hypertension and cardiac fibrosis following prolonged use. The plasma half-life is about five days. It was withdrawn from the market in the UK in 1974. See also * Aminorex * Cericlamine * Cloforex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3,4-dichloroaniline
Dichloroanilines are chemical compounds which consist of an aniline ring substituted with two chlorine atoms and have the molecular formula C6H5Cl2N. There are six isomers, varying in the positions of the chlorine atoms around the ring relative to the amino group. As aniline derivatives, they are named with the amino group in position 1. They are all colorless, although commercial samples can appear colored due to the presence of impurities. Several derivatives are used in the production of dyes and herbicides.Thomas Kahl, Kai-Wilfrid Schröder, F. R. Lawrence, W. J. Marshall, Hartmut Höke, Rudolf Jäckh "Aniline" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2007; John Wiley & Sons: New York. {, class="wikitable" , +Dichloroaniline isomers , - ! Compound name !! CAS# !! Chemical structure !! Melting point , - , 2,3-Dichloroaniline , , 608-27-5 , , , , , - , 2,4-Dichloroaniline , , 554-00-7 , , , , , - , 2,5-Dichloroaniline , , 95-82-9 , , , , , - , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Etolorex
Etolorex is an anorectic of the amphetamine class. It was never marketed. Synthesis Made by the reaction of chlorphentermine with 2-Chloroethanol. See also * 3,4-Dichloroamphetamine * Cericlamine * Chlorphentermine * Cloforex * Clortermine * Methylenedioxyphentermine * Phentermine Phentermine ( phenyl- tertiary-butyl amine), with several brand names including Ionamin and Sentis, is a medication used together with diet and exercise to treat obesity. It is taken by mouth for up to a few weeks at a time, after which the ben ... References Primary alcohols Substituted amphetamines Anorectics Chloroarenes Monoamine releasing agents Abandoned drugs {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorphentermine
Chlorphentermine (trade names Apsedon, Desopimon, Lucofen) is a serotonergic appetite suppressant of the amphetamine family. Developed in 1962, it is the 4-chloro derivative of the better known appetite suppressant phentermine, which is still in current use. Chlorphentermine acts as a highly selective serotonin releasing agent (SRA). It is not a psychostimulant and has little or no abuse potential, but is classed as a Schedule III drug in the USA due mainly to its similarity to other appetite suppressants such as diethylpropion which have been more widely abused. It is no longer used due mainly to safety concerns, as it has a serotonergic effects profile similar to other withdrawn appetite suppressants such as fenfluramine and aminorex which were found to cause pulmonary hypertension and cardiac fibrosis following prolonged use. The plasma half-life is about five days. It was withdrawn from the market in the UK in 1974. See also * Aminorex * Cericlamine * Cloforex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alaproclate
Alaproclate (developmental code name GEA-654) is a drug that was being developed as an antidepressant by the Swedish pharmaceutical company Astra AB (now AstraZeneca) in the 1970s. It acts as a selective serotonin reuptake inhibitor (SSRI), and along with zimelidine and indalpine, was one of the first of its kind. Development was discontinued due to the observation of liver complications in rodent studies. In addition to its SSRI properties, alaproclate has been found to act as a non-competitive NMDA receptor antagonist, but does not have discriminative stimulus properties similar to phencyclidine. Synthesis Method for Treatment of Senile Dementia:Ulf H. A. Lindberg, Sven-Ove gren, (1984 to Astra Lakemedel Aktiebolag). The Grignard reaction between methyl 4-chlorophenylacetate 2449-43-1(1) with methylmagnesium iodide gives 1-(4-chlorophenyl)-2-methyl-2-propanol 468-97-3(2). Acylation with 2-bromopropionyl bromide 63-76-8(3) gives -(4-Chlorophenyl)-2-methylpropan-2-yl2-b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
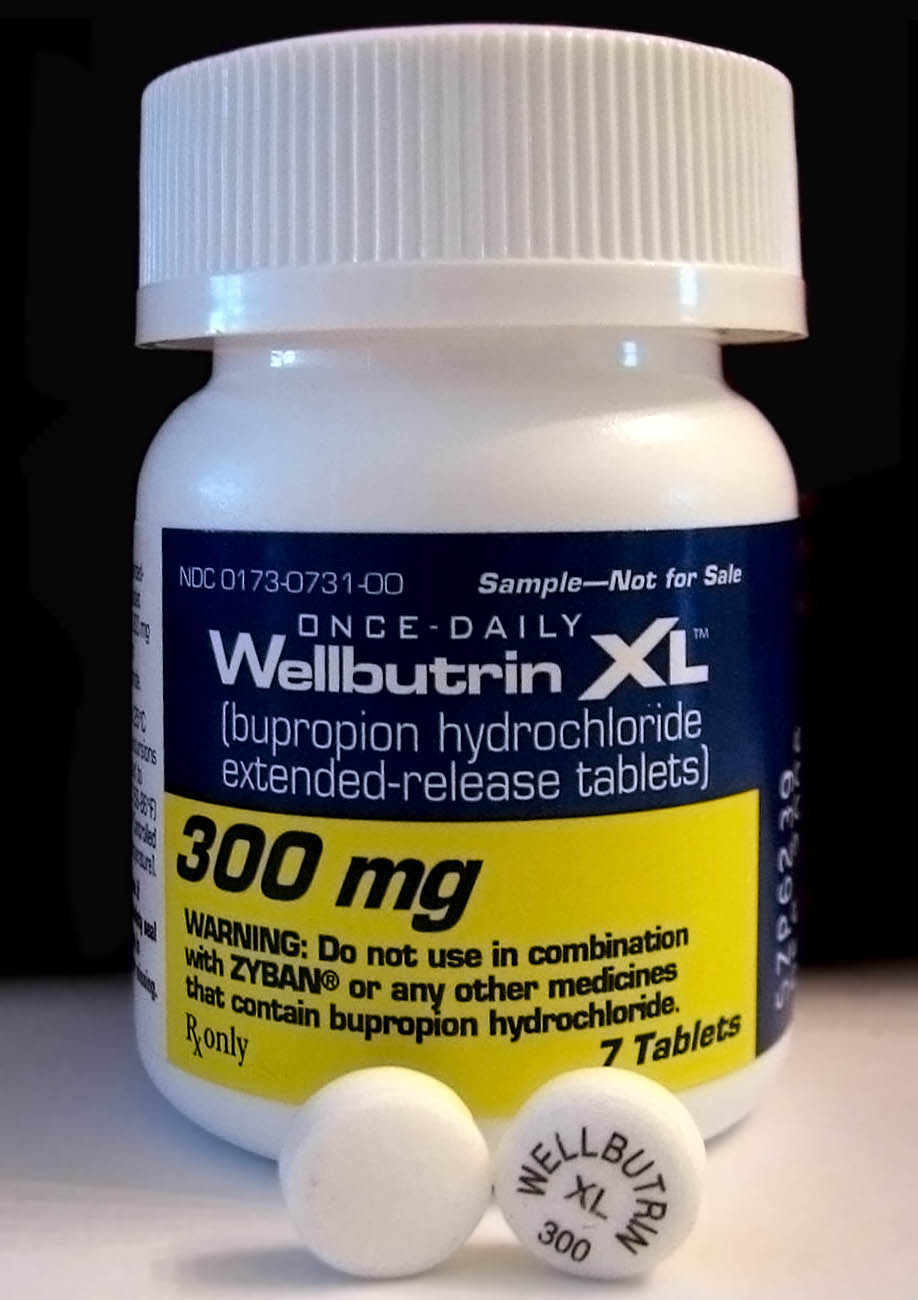
Bupropion
Bupropion, sold under the brand names Wellbutrin and Zyban among others, is an atypical antidepressant primarily used to treat major depressive disorder and to support smoking cessation. It is also popular as an add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant. Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction; it is not associated with weight gain and sleepiness, and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue. Bupropion does, however, carry a much higher risk of seizure than many other antidepressants and extreme caution must be taken in patients with a history of seizure disorder. Common adverse effects of bupropion with the greatest difference from placebo are dry mouth, nausea, constipation, insomnia, anxiety, tremor, and excessive sweating. Raised blood pressure is notable. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |