|
Carbon 13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Isotope
Stable nuclides are Isotope, isotopes of a chemical element whose Nucleon, nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The Atomic nucleus, nuclei of such isotopes are not radioactive and unlike radionuclides do not spontaneously undergo radioactive decay. When these nuclides are referred to in relation to specific elements they are usually called that element's stable isotopes. The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been shown to decay using current equipment. Of these 80 elements, 26 have only one stable isotope and are called monoisotopic element, monoisotopic. The other 56 have more than one stable isotope. Tin has ten stable isotopes, the largest number of any element. Definition of stability, and naturally occurring nuclides Most naturally occurring nuclides are stable (about 251; see list at the end of this article), and about 35 more (tot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopic Signature
An isotopic signature (also isotopic fingerprint) is a ratio of non-radiogenic ' stable isotopes', stable radiogenic isotopes, or unstable radioactive isotopes of particular elements in an investigated material. The ratios of isotopes in a sample material are measured by isotope-ratio mass spectrometry against an isotopic reference material. This process is called isotope analysis. Stable isotopes The atomic mass of different isotopes affect their chemical kinetic behavior, leading to natural isotope separation processes. Carbon isotopes For example, different sources and sinks of methane have different affinity for the 12C and 13C isotopes, which allows distinguishing between different sources by the 13C/12C ratio in methane in the air. In geochemistry, paleoclimatology and paleoceanography this ratio is called δ13C. The ratio is calculated with respect to Pee Dee Belemnite (PDB) standard: :\delta \ce_\mathrm = \left(\frac - 1\right) \cdot 1000 ‰ Similarly, c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen-13
Nitrogen-13 (13N) is a radioisotope of nitrogen used in positron emission tomography (PET). It has a half-life of a little under ten minutes, so it must be made at the PET site. A cyclotron may be used for this purpose. Nitrogen-13 is used to tag ammonia molecules for PET myocardial perfusion imaging. Production Nitrogen-13 is used in medical PET imaging in the form of 13N-labelled ammonia. It can be produced with a medical cyclotron, using a target of pure water with a trace amount of ethanol. The reactants are oxygen-16 (present as H2O) and a proton, and the products are nitrogen-13 and an alpha particle (helium-4). :1H + 16O → 13N + 4He The proton must be accelerated to have total energy greater than 5.66 MeV. This is the threshold energy for this reaction, as it is endothermic (i.e., the mass of the products is greater than the reactants, so energy needs to be supplied which is converted to mass). For this reason, the proton needs to carry extra energy to induce the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
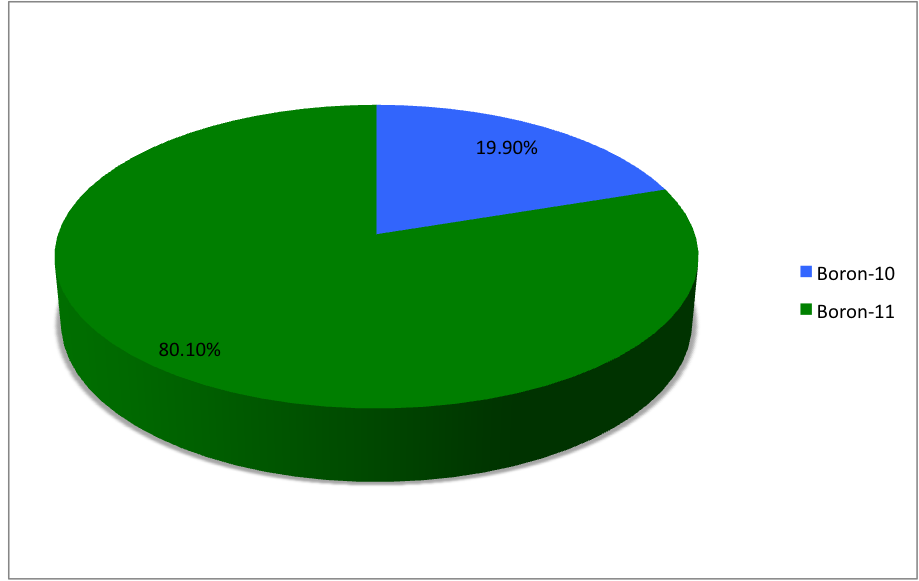
Boron-13
Boron (5B) naturally occurs as isotopes and , the latter of which makes up about 80% of natural boron. There are 13 radioisotopes that have been discovered, with mass numbers from 7 to 21, all with short half-lives, the longest being that of , with a half-life of only and with a half-life of . All other isotopes have half-lives shorter than . Those isotopes with mass below 10 decay into helium (via short-lived isotopes of beryllium for and ) while those with mass above 11 mostly become carbon. List of isotopes , -id=Boron-7 , , style="text-align:center" , 5 , style="text-align:center" , 2 , , [] , p , Subsequently decays by double proton emission to for a net reaction of → + 3 , (3/2−) , , , - , Has 1 halo nucleus, halo protonIntermediate product of Proton–proton chain#The p–p III branch, a branch of proton–proton chain in stellar nucleosynthesis as part of the process converting hydrogen to helium , style="text-align:center" , 5 , style ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Fractionation
Isotope fractionation describes fractionation processes that affect the relative abundance of isotopes, a phenomena that occurs (and so advantage is taken of it) in the study geochemistry, biochemistry, food science, and other fields. Normally, the focus is on stable isotopes of the same element. Isotopic fractionation can be measured by isotope analysis, using isotope-ratio mass spectrometry, nuclear magnetic resonance methods ( specialised techniques,) cavity ring-down spectroscopy, etc., to measure ratios of isotopes, important tools to understand geochemical and biological systems, past and present. For example, biochemical processes cause changes in ratios of stable carbon isotopes incorporated into biomass. Definition Stable isotopes partitioning between two substances ''A'' and ''B'' can be expressed by the use of the isotopic fractionation factor (alpha): : where ''R'' is the ratio of the heavy to light isotope (e.g., 2H/1H or 18O/16O). Values for alpha tend to be v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Carbon
Carbon (6C) has 14 known isotopes, from to as well as , of which only and are stable. The longest-lived radioisotope is , with a half-life of years. This is also the only carbon radioisotope found in nature, as trace quantities are formed cosmogenically by the reaction + → + . The most stable artificial radioisotope is , which has a half-life of . All other radioisotopes have half-lives under 20 seconds, most less than 200 milliseconds. The least stable isotope is , with a half-life of . Light isotopes tend to decay into isotopes of boron and heavy ones tend to decay into isotopes of nitrogen. List of isotopes , -id=Carbon-8 , , style="text-align:right" , 6 , style="text-align:right" , 2 , , [] , proton emission, 2p , Also immediately emits two protons for the net reaction of → + 4 , 0+ , , , -id=Carbon-9 , rowspan=3, , rowspan=3 style="text-align:right" , 6 , rowspan=3 style="text-align:right" , 3 , rowspan=3, , rowspan=3, , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest oxocarbon, carbon oxide. In coordination complexes, the carbon monoxide ligand is called ''metal carbonyl, carbonyl''. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds. Numerous environmental and biological sources generate carbon monoxide. In industry, carbon monoxide is important in the production of many compounds, including drugs, fragrances, and fuels. Indoors CO is one of the most acutely toxic contaminants affecting indoor air quality. CO may be emitted from tobacco smoke and generated from malfunctioning fuel-burning stoves (wood, kerosene, natural gas, propane) and fuel-burning heating systems (wood, oil, n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it is difficult because it is a gas at standard temperature and pressure. In the Earth's atmosphere methane is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Methane is an Organic chemistry, organic Organic compound, compound, and among the simplest of organic compounds. Methane is also a hydrocarbon. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the Atmosphere of Earth, atmosphere, it is known as atmospheric methane. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-14
Carbon-14, C-14, C or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic matter is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (C), which makes up 99% of all carbon on Earth; carbon-13 (C), which makes up 1%; and carbon-14 (C), which occurs in trace amounts, making up about 1-1.5 atoms per 10 atoms of carbon in the atmosphere. C and C are both stable; C is unstable, with half-life years. Carbon-14 has a specific activity of 62.4 mCi/mmol (2.31 GBq/mmol), or 164.9 GBq/g. Carbon-14 decay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
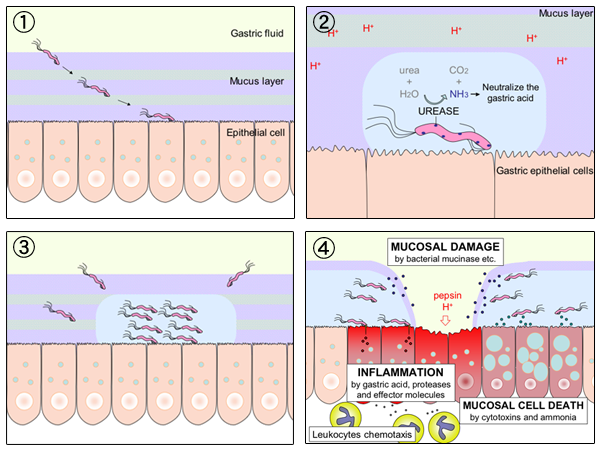
Helicobacter Pylori
''Helicobacter pylori'', previously known as ''Campylobacter pylori'', is a gram-negative, Flagellum#bacterial, flagellated, Bacterial cellular morphologies#Helical, helical bacterium. Mutants can have a rod or curved rod shape that exhibits less virulence. Its Helix, helical body (from which the genus name ''Helicobacter'' derives) is thought to have evolved to penetrate the gastric mucosa, mucous lining of the stomach, helped by its flagella, and thereby establish infection. While many earlier reports of an association between bacteria and the ulcers had existed, such as the works of John Lykoudis, it was only in 1983 when the bacterium was formally described for the first time in the English-language Western literature as the causal agent of peptic ulcer, gastric ulcers by Australian physician-scientists Barry Marshall and Robin Warren. In 2005, the pair was awarded the Nobel Prize in Physiology or Medicine for their discovery. Infection of the stomach with ''H. pylori'' doe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13 Nuclear Magnetic Resonance
Carbon-13 (C13) nuclear magnetic resonance (most commonly known as carbon-13 NMR spectroscopy or 13C NMR spectroscopy or sometimes simply referred to as carbon NMR) is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is analogous to proton NMR ( NMR) and allows the identification of carbon atoms in an organic molecule just as proton NMR identifies hydrogen atoms. 13C NMR detects only the isotope. The main carbon isotope, does not produce an NMR signal. Although ca. 1 mln. times less sensitive than 1H NMR spectroscopy, 13C NMR spectroscopy is widely used for characterizing organic and organometallic compounds, primarily because 1H-decoupled 13C-NMR spectra are more simple, have a greater sensitivity to differences in the chemical structure, and, thus, are better suited for identifying molecules in complex mixtures. At the same time, such spectra lack quantitative information about the atomic ratios of different types of carbon nuclei, because nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Permian Extinction
The Permian ( ) is a geologic period and stratigraphic system which spans 47 million years, from the end of the Carboniferous Period million years ago (Mya), to the beginning of the Triassic Period 251.902 Mya. It is the sixth and last period of the Paleozoic Era; the following Triassic Period belongs to the Mesozoic Era. The concept of the Permian was introduced in 1841 by geologist Sir Roderick Murchison, who named it after the region of Perm in Russia. The Permian witnessed the diversification of the two groups of amniotes, the synapsids and the sauropsids (reptiles). The world at the time was dominated by the supercontinent Pangaea, which had formed due to the collision of Euramerica and Gondwana during the Carboniferous. Pangaea was surrounded by the superocean Panthalassa. The Carboniferous rainforest collapse left behind vast regions of desert within the continental interior. Amniotes, which could better cope with these drier conditions, rose to dominance in place of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |