|
Carbohydrate Synthesis
Carbohydrate synthesis is a sub-field of organic chemistry concerned with generating complex carbohydrate structures from simple units (monosaccharides). The generation of carbohydrate structures usually involves linking monosaccharides or oligosaccharides through glycosidic bonds, a process called glycosylation. Therefore, it is important to construct glycosidic linkages that have optimum molecular geometry (stereoselectivity) and the stable bond (regioselectivity) at the reaction site (anomeric centre). Background Carbohydrates can generally be classified into one of two groups, Monosaccharide, monosacharides, and complex carbohydrates. Monosacharides (also called "simple sugars") are the simplest single units of any carbohydrate; the most common monosaccharides are five and six carbon compounds such as glucose, fructose, and galactose. Complex carbohydrates are combinations of monosaccarides linked together through connections called Glycosidic bond, glycosidic bonds, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolysis Pathway 2
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and NADH, reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes. The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, can occur in the Great Oxygenation Event, oxygen-free conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal ions, meaning this is a plausible prebiotic pathway for abiogenesis. The most common type of glycolysis is the ''Embden–Meyerhof–Parnas (EMP) pathway'', which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Kar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycal
Glycal is a name for cyclic enol ether derivatives of sugars having a double bond between carbon atoms 1 and 2 of the ring. The term "glycal" should not be used for an unsaturated sugar that has a double bond in any position other than between carbon atoms 1 and 2. History The first glycal was synthesized by Hermann Emil Fischer and Karl Zach in 1913. They synthesized this 1,2-unsaturated sugar from D-glucose and named their product D-glucal. Fischer believed he had synthesized an aldehyde, and therefore he gave the product a name that suggested this. By the time he discovered his mistake, the name "glycal" was adopted as a general name for all sugars with a double bond between carbon atoms 1 and 2. Conformation Glycals can be formed as pyranose (six-membered) or furanose (five-membered) rings, depending on the monosaccharide used as a starting material to synthesize the glycal. Glycals can also be classified as ''endo''-glycals or ''exo''-glycals. A glycal is an endo-glyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioglycosides
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an ''O-glycoside''), N- (a ''glycosylamine''), S-(a ''thioglycoside''), or C- (a ''C-glycoside'') glycosidic bond. According to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosyl Halide
In organic chemistry, a glycosyl group is a univalent free radical or substituent structure obtained by removing the hydroxyl () group from the hemiacetal () group found in the cyclic form of a monosaccharide and, by extension, of a lower oligosaccharide. Glycosyl groups are exchanged during glycosylation from the glycosyl donor, the electrophile, to the glycosyl acceptor, the nucleophile. The outcome of the glycosylation reaction is largely dependent on the reactivity of each partner. Glycosyl also reacts with inorganic acids, such as phosphoric acid, forming an ester such as glucose 1-phosphate. Examples In cellulose, glycosyl groups link together 1,4-β-D-glucosyl units to form chains of (1,4-β-D-glucosyl)n. Other examples include ribityl in 6,7-Dimethyl-8-ribityllumazine, and glycosylamines. Alternative substituent groups Instead of the hemiacetal hydroxyl group, a ''hydrogen'' atom can be removed to form a substituent, for example the hydrogen from the C3 hydroxyl of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosyl Acceptor
A glycosyl acceptor is any suitable nucleophile-containing molecule that will react with a glycosyl donor to form a new glycosidic bond. By convention, the acceptor is the member of this pair which did not contain the resulting anomeric carbon of the new glycosidic bond. Since the nucleophilic atom of the acceptor is typically an oxygen atom, this can be remembered using the mnemonic of ''the acceptor is the alcohol.'' A glycosyl acceptor can be a mono- or oligosaccharide that contains an available nucleophile, such as an unprotected hydroxyl. Background Examples glucose to haemoglobin See also * Chemical glycosylation * Glycosyl halide * Armed and disarmed saccharides * Carbohydrate chemistry A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ma ... References *{{cite journal , l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosyl Donor
A glycosyl donor is a carbohydrate mono- or oligosaccharide that will react with a suitable glycosyl acceptor to form a new glycosidic bond. By convention, the donor is the member of this pair that contains the resulting anomeric carbon of the new glycosidic bond.T. K. Lindhorst "Essentials of Carbohydrate Chemistry and Biochemistry" 2007 Wiley-VCH Verlag, Weinheim The resulting reaction is referred to as a glycosylation or chemical glycosylation. In a glycosyl donor, a leaving group is required at the anomeric position. The simplest leaving group is the OH group that is naturally present in monosaccharides, but it requires activation by acid catalysis in order to function as leaving group (in the Fischer glycosylation). More effective leaving groups are in general used in the glycosyl donors employed in chemical synthesis of glycosides. Typical leaving groups are halides, thioalkyl groups, or imidates, but acetate, phosphate, and O-pentenyl groups are also employed. Natural glycosyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined as having the same molecular formula and sequence of bonded atoms (constitution) but differing in the geometric positioning of the atoms in space. For this reason, it is also known as Three-dimensional space, 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry applies to all kinds of compounds and ions, Organic chemistry, organic and Inorganic chemistry, inorganic species alike. Stereochemistry affects Biochemistry, biological, Physical chemistry, physical, and supramolecular chemistry. Stereochemistry reactivity (chemistry), reactivity of the molecules in question (dynamic stereochemistry). History In 1815, Jean-Baptiste Biot's observation of optical activity marked the beginning of organic stereochemistr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anomer
In carbohydrate chemistry, a pair of anomers () is a pair of near-identical stereoisomers or diastereomers that differ at only the anomeric carbon, the carbon atom that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order for anomers to exist, the sugar must be in its cyclic form, since in open-chain form, the anomeric carbon atom is planar and thus achiral. More formally stated, then, an anomer is an epimer at the hemiacetal/hemiketal carbon atom in a cyclic saccharide. Anomerization is the process of conversion of one anomer to the other. As is typical for stereoisomeric compounds, different anomers have different physical properties, melting points and specific rotations. Nomenclature Every two anomers are designated alpha (α) or beta (β), according to the configurational relationship between the ''anomeric centre'' and the ''anomeric reference atom'', hence they are relative stereodescriptors. The anomeric centre in hemiac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
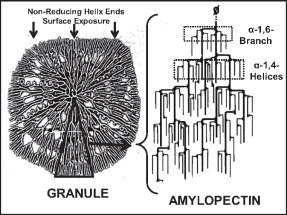
Amylopectin
Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose. Plants store starch within specialized organelles called amyloplasts. To generate energy, the plant hydrolyzes the starch, releasing the glucose subunits. Humans and other animals that eat plant foods also use amylase, an enzyme that assists in breaking down amylopectin, to initiate the hydrolysis of starch. Starch is made of about 70–80% amylopectin by weight, though it varies depending on the source. For example, it ranges from lower percent content in long-grain rice, amylomaize, and Russet Burbank potato, russet potatoes to 100% in glutinous rice, waxy potato starch, and waxy corn. Amylopectin is highly branched, being formed of 2,000 to 200,000 glucose units. Its inner chains are formed of 20–24 glucose subunits. Starch gelatinization, Dissolved amylopectin starch has a lower tendency of retrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
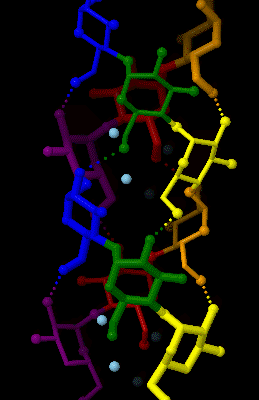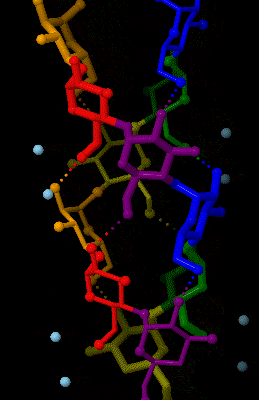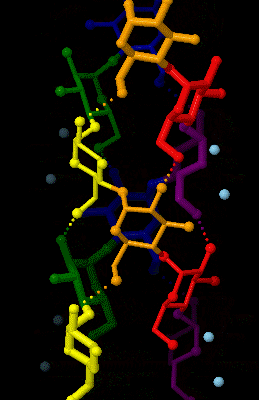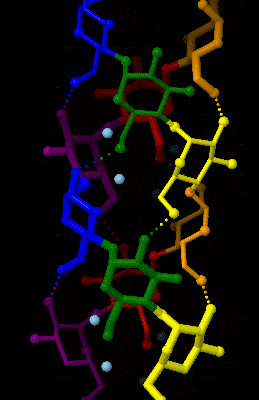
Amylose
Amylose is a polysaccharide made of α-D-glucose units, bonded to each other through α(1→4) glycosidic bonds. It is one of the two components of starch, making up approximately 20–25% of it. Because of its tightly packed Helix, helical structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch. Structure Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The structural formula of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands. There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation, found both in starch granules and in hydrated amylose (when starch i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. It is the main storage form of glucose in the human body. Glycogen functions as one of three regularly used forms of energy reserves, creatine phosphate being for very short-term, glycogen being for short-term and the triglyceride stores in adipose tissue (i.e., body fat) being for long-term storage. Protein, broken down into amino acids, is seldom used as a main energy source except during starvation and glycolytic crisis ''(see bioenergetic systems)''. In humans, glycogen is made and stored primarily in the cells of the liver and skeletal muscle. In the liver, glycogen can make up 5–6% of the organ's fresh weight: the liver of an adult, weighing 1.5 kg, can store roughly 100–120 grams of glycogen. In skeletal muscle, glycogen is found in a low concentration (1–2% of the muscle mass): the skeletal muscle of an adult weighing 70 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |