|
Calponin Structure Wikigene Final
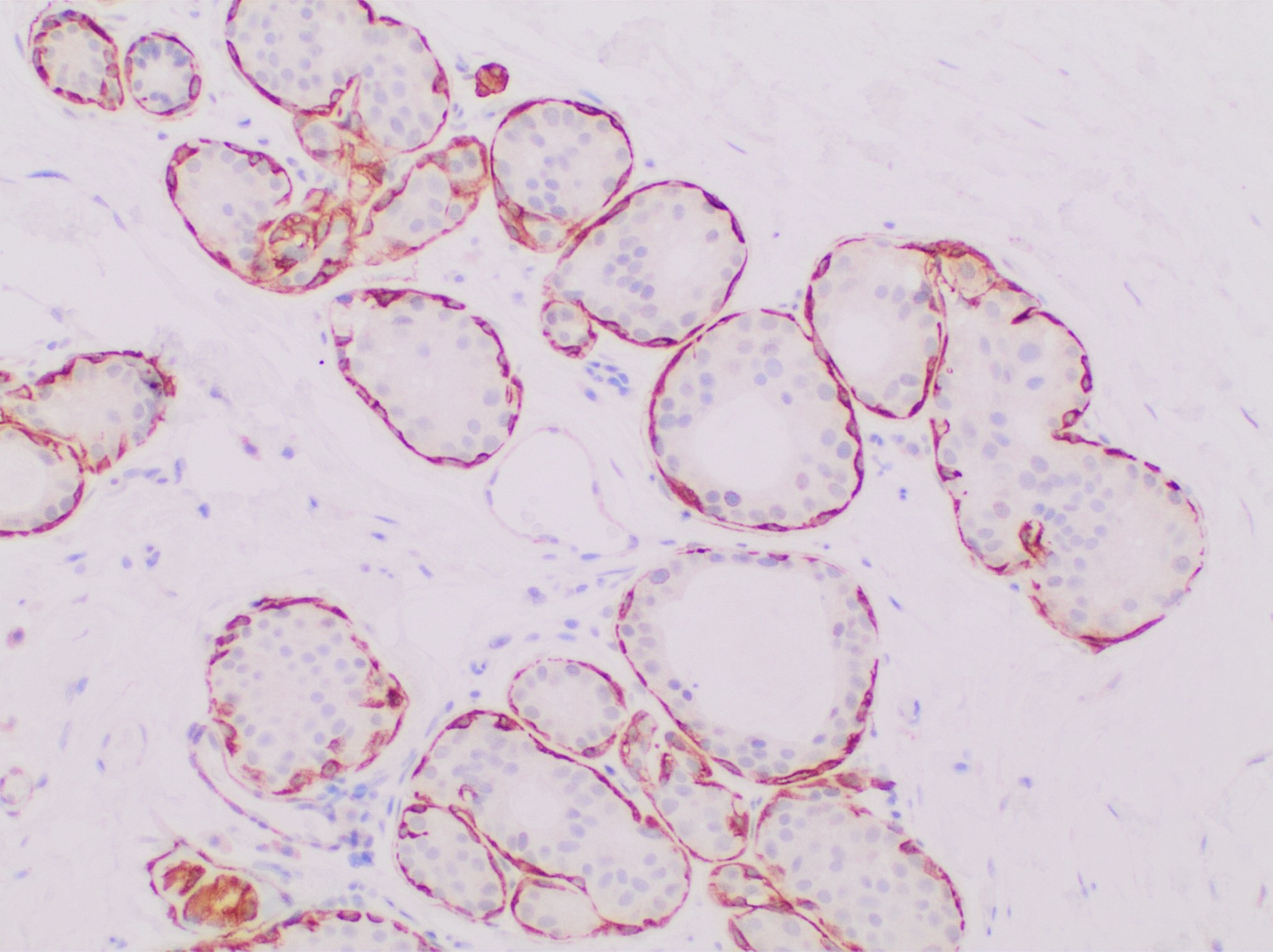
Calponin is a calcium binding protein. Calponin tonically inhibits the ATPase activity of myosin in smooth muscle. Phosphorylation of calponin by a protein kinase, which is dependent upon calcium binding to calmodulin, releases the calponin's inhibition of the smooth muscle ATPase. Structure and function Calponin is mainly made up of α-helices with hydrogen bond turns. It is a binding protein and is made up of three domains. These domains in order of appearance are Calponin Homology (CH), regulatory domain (RD), and Click-23, domain that contains the calponin repeats. At the CH domain calponin binds to α-actin and filamin and binds to actin within the RD domain. Calmodulin, when activated by calcium may bind weakly to the CH domain and inhibit calponin binding with α-actin. Calponin is responsible for binding many actin binding proteins, phospholipids, and regulates the actin/myosin interaction. Calponin is also thought to negatively affect the bone making process due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules such as proteins and nucleic acids, which is overseen by the Worldwide Protein Data Bank (wwPDB). This structural data is obtained and deposited by biologists and biochemists worldwide through the use of experimental methodologies such as X-ray crystallography, Nuclear magnetic resonance spectroscopy of proteins, NMR spectroscopy, and, increasingly, cryo-electron microscopy. All submitted data are reviewed by expert Biocuration, biocurators and, once approved, are made freely available on the Internet under the CC0 Public Domain Dedication. Global access to the data is provided by the websites of the wwPDB member organizations (PDBe, PDBj, RCSB PDB, and BMRB). The PDB is a key in areas of structural biology, such as structural genomics. Most major scientific journals and some funding agencies now require scientists to submit their structure data to the PDB. Many other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osteoblast
Osteoblasts (from the Greek combining forms for " bone", ὀστέο-, ''osteo-'' and βλαστάνω, ''blastanō'' "germinate") are cells with a single nucleus that synthesize bone. However, in the process of bone formation, osteoblasts function in groups of connected cells. Individual cells cannot make bone. A group of organized osteoblasts together with the bone made by a unit of cells is usually called the osteon. Osteoblasts are specialized, terminally differentiated products of mesenchymal stem cells. They synthesize dense, crosslinked collagen and specialized proteins in much smaller quantities, including osteocalcin and osteopontin, which compose the organic matrix of bone. In organized groups of disconnected cells, osteoblasts produce hydroxyapatite, the bone mineral, that is deposited in a highly regulated manner, into the inorganic matrix forming a strong and dense mineralized tissue, the mineralized matrix. Hydroxyapatite-coated bone implants often perfo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calmodulin
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all Eukaryote, eukaryotic cells. It is an intracellular target of the Second messenger system, secondary messenger Calcium in biology, Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium Signal transduction, signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases. Structure Calmodulin is a small, highly conserved protein that is 148 amino acids long (16.7 kDa). The protein has two approximately symmetrical globular domains (the N- and C- domains) each containing a pair of EF hand Sequence motif, motifs separated by a flexible linker region for a total of four Ca2+ binding sites, two in each globular domain. In the Ca2+-free state, the helices that form the four EF-hands are collapsed in a co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calponin Family Repeat
In molecular biology, the calponin family repeat is a 26 amino acid protein domain. Calponin 1 (CNN1) contains three copies of this domain. This domain is also found in vertebrate smooth muscle protein (SM22 or transgelin), and a number of other proteins whose physiological role is not yet established, including ''Drosophila'' synchronous flight muscle protein SM20, ''Caenorhabditis elegans'' unc-87 protein, rat neuronal protein NP25, and an ''Onchocerca volvulus'' antigen In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. .... References {{InterPro content, IPR000557 Protein domains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
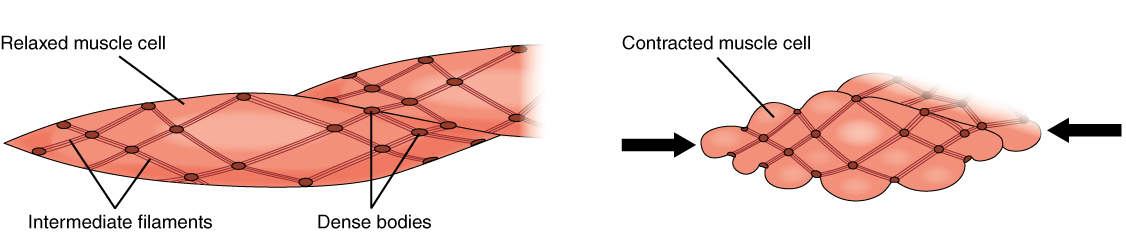
Smooth Muscle
Smooth muscle is one of the three major types of vertebrate muscle tissue, the others being skeletal and cardiac muscle. It can also be found in invertebrates and is controlled by the autonomic nervous system. It is non- striated, so-called because it has no sarcomeres and therefore no striations (''bands'' or ''stripes''). It can be divided into two subgroups, ''single-unit'' and ''multi-unit'' smooth muscle. Within single-unit muscle, the whole bundle or sheet of smooth muscle cells contracts as a syncytium. Smooth muscle is found in the walls of hollow organs, including the stomach, intestines, bladder and uterus. In the walls of blood vessels, and lymph vessels, (excluding blood and lymph capillaries) it is known as vascular smooth muscle. There is smooth muscle in the tracts of the respiratory, urinary, and reproductive systems. In the eyes, the ciliary muscles, iris dilator muscle, and iris sphincter muscle are types of smooth muscles. The iris dilator and s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calmodulin
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all Eukaryote, eukaryotic cells. It is an intracellular target of the Second messenger system, secondary messenger Calcium in biology, Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium Signal transduction, signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases. Structure Calmodulin is a small, highly conserved protein that is 148 amino acids long (16.7 kDa). The protein has two approximately symmetrical globular domains (the N- and C- domains) each containing a pair of EF hand Sequence motif, motifs separated by a flexible linker region for a total of four Ca2+ binding sites, two in each globular domain. In the Ca2+-free state, the helices that form the four EF-hands are collapsed in a co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinase
In biochemistry, a kinase () is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. As a result, kinase produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group (producing a dephosphorylated substrate and the high energy molecule of ATP). These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis. Kinases are part of the larger family of phosphotransferases. Kinases should not be confused with phosphorylases, which catalyze the addition of inorganic phosphate groups to an acceptor, nor with phosphatases, which remove phosphate groups (dephosphorylation). The phosphorylation state of a molecule, whether it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossils of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name comes from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceuticals for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calponin 1
Calponin 1 is a basic smooth muscle protein that in humans is encoded by the ''CNN1'' gene. The ''CNN1'' gene is located at 19p13.2-p13.1 in the human chromosomal genome and contains 7 exons, encoding the protein calponin 1, an actin filament-associated regulatory protein. Human calponin 1 is a 33.2-KDa protein consists of 297 amino acids with an isoelectric point of 9.1, thus calponin 1 is also known as basic calponin. Evolution Three homologous genes, ''Cnn1'', ''Cnn2'' and ''Cnn3'', have evolved in vertebrates, encoding three isoforms of calponin: calponin 1, calponin 2, calponin 3, respectively. Protein sequence alignment shows that calponin 1 is highly conserved in mammals but more diverged among lower vertebrates. Smooth muscle-specific expression The expression of CNN1 is specific to differentiated mature smooth muscle cells, suggesting a role in contractile functions. Calponin 1 is up-regulated in smooth muscle tissues during postnatal development with a higher conte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CNN3 (gene)
Calponin 3. acidic is a protein that in humans is encoded by the ''CNN3'' gene. The ''CNN3'' gene is located at 1p22-p21 in the human chromosomal genome. ''CNN3'' gene contains 7 exons and encodes calponin 3, a 36.4-kDa protein consisting of 329 amino acids with isoelectric point (pI) of 5.84. Calponin 3 is known as acidic calponin. Among three isoforms of calponin, less is known for the gene regulation and function of calponin 3. Nonetheless, much has been learned from extensive studies on the homologous genes ''CNN1'' and ''CNN2'' that encode calponin 1 and calponin 2. Evolution ''CNN3'' is one of the three homologous calponin isoform genes. Calponin 3 is significantly diverged from calponin 1 and calponin 2 in the C terminal variable region. The higher degree of divergence among vertebrate ''CNN3'' genes than that in the ''CNN1'' and ''CNN2'' gene families suggests possibly earlier emergence of ''CNN3'', indicating that calponin 3 may represent a prototype of calponin ance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calponin 3
Calponin is a calcium binding protein. Calponin tonically inhibits the ATPase activity of myosin in smooth muscle. Phosphorylation of calponin by a protein kinase, which is dependent upon calcium binding to calmodulin, releases the calponin's inhibition of the smooth muscle ATPase. Structure and function Calponin is mainly made up of α-helices with hydrogen bond turns. It is a binding protein and is made up of three domains. These domains in order of appearance are Calponin Homology (CH), regulatory domain (RD), and Click-23, domain that contains the calponin repeats. At the CH domain calponin binds to α-actin and filamin and binds to actin within the RD domain. Calmodulin, when activated by calcium may bind weakly to the CH domain and inhibit calponin binding with α-actin. Calponin is responsible for binding many actin binding proteins, phospholipids, and regulates the actin/myosin interaction. Calponin is also thought to negatively affect the bone making process due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |