|
CYP2D6
Cytochrome P450 2D6 (CYP2D6) is an enzyme that in humans is encoded by the ''CYP2D6'' gene. ''CYP2D6'' is primarily expressed in the liver. It is also highly expressed in areas of the central nervous system, including the substantia nigra. CYP2D6, a member of the cytochrome P450 mixed-function oxidase system, is one of the most important enzymes involved in the metabolism of xenobiotics in the body. In particular, CYP2D6 is responsible for the metabolism and elimination of approximately 25% of clinically used drugs, via the addition or removal of certain functional groups – specifically, hydroxylation, demethylation, and dealkylation. CYP2D6 also activates some prodrugs. This enzyme also metabolizes several endogenous substances, such as N,N-Dimethyltryptamine, hydroxytryptamines, neurosteroids, and both ''m''-tyramine and ''p''-tyramine which CYP2D6 metabolizes into dopamine in the brain and liver. Considerable variation exists in the efficiency and amount of C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Codeine
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, ''Papaver somniferum''. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children. In Europe, it is not recommended as a cough medicine for those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of addiction and overdose. Common side effects include nausea, vomiting, constipation, itchiness, lightheadedness, and drowsiness. Serious ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tramadol
Tramadol, sold under the brand name Tramal among others, is an opioid analgesic, pain medication and a serotonin–norepinephrine reuptake inhibitor (SNRI) used to treat moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It is available in combination with paracetamol (acetaminophen). As is typical of opioids, common side effects include constipation, pruritis, itchiness, and nausea. Serious side effects may include hallucinations, seizures, increased risk of serotonin syndrome, decreased alertness, and drug addiction. A change in dosage may be recommended in those with chronic kidney disease, kidney or liver problems. It is not recommended in those who are at risk of suicide or in those who are pregnant. While not recommended in women who are breastfeeding, those who take a single dose should not generally have to stop breastfeeding. Tramadol is converted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
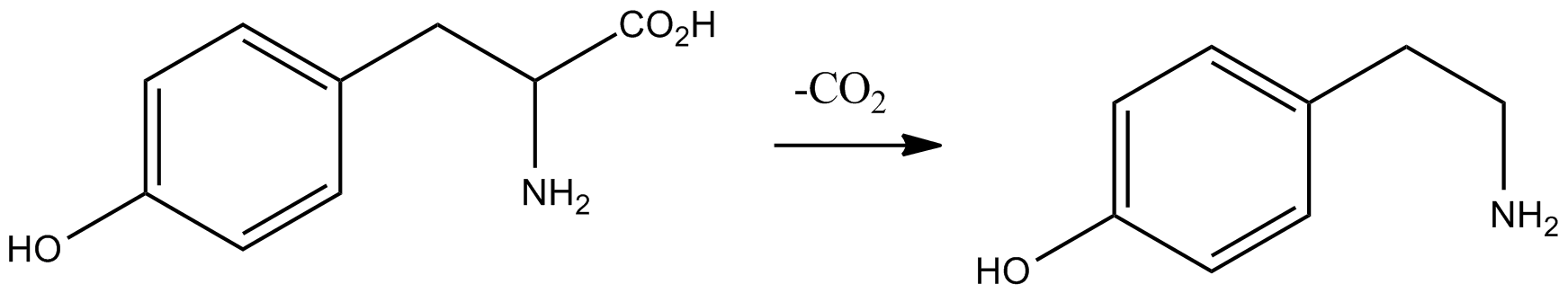
M-tyramine
''meta''-Tyramine, also known as ''m''-tyramine and 3-tyramine, as well as 3-hydroxyphenethylamine, is an endogenous trace amine neuromodulator and a structural analog of phenethylamine. It is a positional isomer of ''para''-tyramine, and similarly to it, has effects on the adrenergic and dopaminergic systems. ''meta''-Tyramine is produced in humans via aromatic amino acid decarboxylase-mediated metabolism of ''meta''-tyrosine. meta-Tyramine can be metabolized into dopamine via peripheral or brain CYP2D6 enzymes in humans. See also * ''para''-Tyramine * 3-Methoxytyramine 3-Methoxytyramine (3-MT), also known as 3-methoxy-4-hydroxyphenethylamine, is a human trace amine and the major metabolite of the monoamine neurotransmitter dopamine. It is formed by the introduction of a methyl group to dopamine by the enzyme c ... * 3-Hydroxy-''N'',''N''-dimethylphenethylamine (LSM-6) References Phenethylamines 3-Hydroxyphenyl compounds TAAR1 agonists Trace amines Nore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and many addictive drugs increase dopamine release or block its reuptake into neurons following release. Other brain dopamine pathways are involved in motor control and in controllin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N,N-Dimethyltryptamine
Dimethyltryptamine (DMT), also known as ''N'',''N''-dimethyltryptamine (''N'',''N''-DMT), is a serotonergic hallucinogen and investigational drug of the tryptamine family that occurs naturally in many plants and animals, including humans. DMT is used as a psychedelic drug and prepared by various cultures for ritual purposes as an entheogen. DMT has a rapid onset, intense effects, and a relatively short duration of action. For those reasons, DMT was known as the "businessman's trip" during the 1960s in the United States, as a user could access the full depth of a psychedelic experience in considerably less time than with other substances such as LSD or psilocybin mushrooms. DMT can be inhaled or injected and its effects depend on the dose, as well as the mode of administration. When inhaled or injected, the effects last about five to fifteen minutes. Effects can last three hours or more when orally ingested along with a monoamine oxidase inhibitor (MAOI), such as the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyramine
Tyramine ( ) (also spelled tyramin), also known under several other names, is a naturally occurring trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with the use of monoamine oxidase inhibitors (MAOIs). Occurrence Tyramine occurs widely in plants and animals, and is metabolized by various enzymes, including monoamine oxidases. In foods, it often is produced by the decarboxylation of tyrosine during fermentation or decay. Foods that are fermented, cured, pickled, aged, or spoiled have high amounts of tyramine. Tyramine levels go up when foods are at room temperature or go past their freshness date. Specific foods containing considerable amounts of tyramine include: * Strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Interaction
In pharmaceutical sciences, drug interactions occur when a drug's mechanism of action is affected by the concomitant administration of substances such as foods, beverages, or other drugs. A popular example of drug–food interaction is the effect of grapefruit on the metabolism of drugs. Interactions may occur by simultaneous targeting of receptors, directly or indirectly. For example, both Zolpidem and alcohol affect GABAA receptors, and their simultaneous consumption results in the overstimulation of the receptor, which can lead to loss of consciousness. When two drugs affect each other, it is a drug–drug interaction (DDI). The risk of a DDI increases with the number of drugs used. A large share of elderly people regularly use five or more medications or supplements, with a significant risk of side-effects from drug–drug interactions. Drug interactions can be of three kinds: * additive (the result is what you expect when you add together the effect of each drug take ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catarrhine
The parvorder Catarrhini (known commonly as catarrhine monkeys, Old World anthropoids, or Old World monkeys) consists of the Cercopithecoidea and apes (Hominoidea). In 1812, Geoffroy grouped those two groups together and established the name Catarrhini, "Old World monkeys", ("''singes de l'Ancien Monde''" in French). Its sister in the infraorder Simiiformes is the parvorder Platyrrhini (New World monkeys). There has been some resistance to directly designate apes (and thus humans) as monkeys despite the scientific evidence, so "Old World monkey" may be taken to mean the Cercopithecoidea or the Catarrhini. That apes are monkeys was already realized by Georges-Louis Leclerc, Comte de Buffon in the 18th century. Linnaeus placed this group in 1758 together with what we now recognise as the tarsiers and the New World monkeys, in a single genus "''Simia''" (sans ''Homo''). The Catarrhini are all native to Africa and Asia. Members of this parvorder are called catarrhines. The Catarrh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudogene
Pseudogenes are nonfunctional segments of DNA that resemble functional genes. Pseudogenes can be formed from both protein-coding genes and non-coding genes. In the case of protein-coding genes, most pseudogenes arise as superfluous copies of functional genes, either directly by gene duplication or indirectly by Reverse transcriptase, reverse transcription of an mRNA transcript. Pseudogenes are usually identified when genome sequence analysis finds gene-like sequences that lack regulatory sequences or are incapable of producing a functional product. Pseudogenes are a type of junk DNA. Most non-bacterial genomes contain many pseudogenes, often as many as functional genes. This is not surprising, since various biological processes are expected to accidentally create pseudogenes, and there are no specialized mechanisms to remove them from genomes. Eventually pseudogenes may be deleted from their genomes by chance of DNA replication or DNA repair errors, or they may accumulate so many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromosome 22
Chromosome 22 is one of the 23 pairs of chromosomes in human cells. Humans normally have two copies of chromosome 22 in each cell. Chromosome 22 is the second smallest human chromosome, spanning about 51 million DNA base pairs and representing between 1.5 and 2% of the total DNA in cells. In 1999, researchers working on the Human Genome Project announced they had determined the sequence of base pairs that make up this chromosome. Chromosome 22 was the first human chromosome to be fully sequenced. Human chromosomes are numbered by their apparent size in the karyotype, with chromosome 1 being the largest and chromosome 22 having originally been identified as the smallest. However, genome sequencing has revealed that chromosome 21 is actually smaller than chromosome 22. Genes Number of genes The following are some of the gene count estimates of human chromosome 22. Because researchers use different approaches to genome annotation, their predictions of the number of genes on e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inducer
In molecular biology, an inducer is a molecule that regulates gene expression. An inducer functions in two ways; namely: *By disabling repressors. The gene is expressed because an inducer binds to the repressor. The binding of the inducer to the repressor prevents the repressor from binding to the operator. RNA polymerase can then begin to transcribe operon genes. *By binding to activators. Activators generally bind poorly to activator DNA sequences unless an inducer is present. Activator binds to an inducer and the complex binds to the activation sequence and activates target gene. Removing the inducer stops transcription. Because a small inducer molecule is required, the increased expression of the target gene is called induction. The lactose operon is one example of an inducible system. Function Repressor proteins bind to the DNA strand and prevent RNA polymerase from being able to attach to the DNA and synthesize mRNA. Inducers bind to repressors, causing them to change sha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |