|
COMT
Catechol-''O''-methyltransferase (COMT; ) is one of several enzymes that degrade catecholamines (neurotransmitters such as dopamine, epinephrine, and norepinephrine), catecholestrogens, and various drugs and substances having a catechol structure. In humans, catechol-''O''-methyltransferase protein is encoded by the COMT gene. Two isoforms of COMT are produced: the soluble short form (S-COMT) and the membrane bound long form (MB-COMT). As the regulation of catecholamines is impaired in a number of medical conditions, several pharmaceutical drugs target COMT to alter its activity and therefore the availability of catecholamines. COMT was first discovered by the biochemist Julius Axelrod in 1957. Function Catechol-''O''-methyltransferase is involved in the inactivation of the catecholamine neurotransmitters (dopamine, epinephrine, and norepinephrine). The enzyme introduces a methyl group to the catecholamine, which is donated by S-adenosyl methionine (SAM). Any compound havin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entacapone
Entacapone, sold under the brand name Comtan among others, is a medication commonly used in combination with other medications for the treatment of Parkinson's disease. Entacapone together with levodopa and carbidopa allows levodopa to have a longer effect in the brain and reduces Parkinson's disease signs and symptoms for a greater length of time than levodopa and carbidopa therapy alone. Entacapone is a selective and reversible inhibitor of the enzyme catechol-''O''-methyltransferase (COMT). When taken together with levodopa (L-DOPA) and carbidopa, entacapone stops COMT from breaking down levodopa, resulting in an overall increase of levodopa remaining in the brain and body. Entacapone does not cross into the brain and hence does not inhibit COMT there. Carbidopa/levodopa/entacapone (Stalevo), a medication developed by Orion Pharma and marketed by Novartis, is a single tablet formulation that contains levodopa, carbidopa, and entacapone. Medical uses Entacapone is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catecholamine
A catecholamine (; abbreviated CA), most typically a 3,4-dihydroxyphenethylamine, is a monoamine neurotransmitter, an organic compound that has a catechol (benzene with two hydroxyl side groups next to each other) and a side-chain amine. Catechol can be either a free molecule or a substituent of a larger molecule, where it represents a 1,2-dihydroxybenzene group. Catecholamines are derived from the amino acid tyrosine, which is derived from dietary sources as well as synthesis from phenylalanine. Catecholamines are water-soluble and are 50% bound to plasma proteins in circulation. Included among catecholamines are epinephrine (adrenaline), norepinephrine (noradrenaline), and dopamine. Release of the hormones epinephrine and norepinephrine from the adrenal medulla of the adrenal glands is part of the fight-or-flight response. Tyrosine is created from phenylalanine by hydroxylation by the enzyme phenylalanine hydroxylase. Tyrosine is also ingested directly from dietary prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and many addictive drugs increase dopamine release or block its reuptake into neurons following release. Other brain dopamine pathways are involved in motor control and in controllin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parkinson's Disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor system, motor and non-motor systems. Symptoms typically develop gradually and non-motor issues become more prevalent as the disease progresses. The motor symptoms are collectively called parkinsonism and include tremors, bradykinesia, spasticity, rigidity as well as postural instability (i.e., difficulty maintaining balance). Non-motor symptoms develop later in the disease and include behavior change (individual), behavioral changes or mental disorder, neuropsychiatric problems such as sleep abnormalities, psychosis, anosmia, and mood swings. Most Parkinson's disease cases are idiopathic disease, idiopathic, though contributing factors have been identified. Pathophysiology involves progressive nerve cell death, degeneration of nerve cells in the substantia nigra, a midbrain region that provides dopamine to the basal ganglia, a system invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DOPAC
3,4-Dihydroxyphenylacetic acid (DOPAC) is a metabolite of the neurotransmitter dopamine. Dopamine can be metabolized into one of three substances. One such substance is DOPAC. Another is 3-methoxytyramine (3-MT). Both of these substances are degraded to form homovanillic acid (HVA). Both degradations involve the enzymes monoamine oxidase (MAO) and catechol-O-methyl transferase (COMT), albeit in reverse order: MAO catalyzes dopamine to DOPAC, and COMT catalyzes DOPAC to HVA; whereas COMT catalyzes dopamine to 3-MT and MAO catalyzes 3-MT to HVA. The third metabolic end-product of dopamine is norepinephrine (noradrenaline). It can also be found in the bark of ''Eucalyptus globulus''. This product has been synthesized (52% yield) from 4-hydroxyphenylacetic acid via aerobic biotransformation Biotransformation is the biochemical modification of one chemical compound or a mixture of chemical compounds. Biotransformations can be conducted with whole cells, their lysates, or purif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catecholestrogen
A catechol estrogen is a steroidal estrogen that contains catechol (1,2-dihydroxybenzene) within its chemical structure, structure. The catechol estrogens are endogenous metabolites of estradiol and estrone and include the following compounds: * 2-Hydroxylated: ** 2-Hydroxyestradiol ** 2-Hydroxyestrone ** 2-Hydroxyestriol * 4-Hydroxylated: ** 4-Hydroxyestradiol ** 4-Hydroxyestrone ** 4-Hydroxyestriol The most abundant catechol estrogen in serum and urine is 2-hydroxyestrone, with 2-hydroxyestradiol and 2-hydroxyestriol also being formed, while the principal 4-hydroxy catechol estrogen, 4-hydroxyestrone, is present in only small amounts in urine. 4-Hydroxyestriol has been detected in the urine of pregnancy, pregnant women. The catechol estrogens are formed from estradiol and estrone by cytochrome P450 enzymes predominantly in the liver but also in extrahepatic tissues, and are metabolism, metabolized by catechol O-methyltransferase (COMT) into methoxylated estrogens such as 2-met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
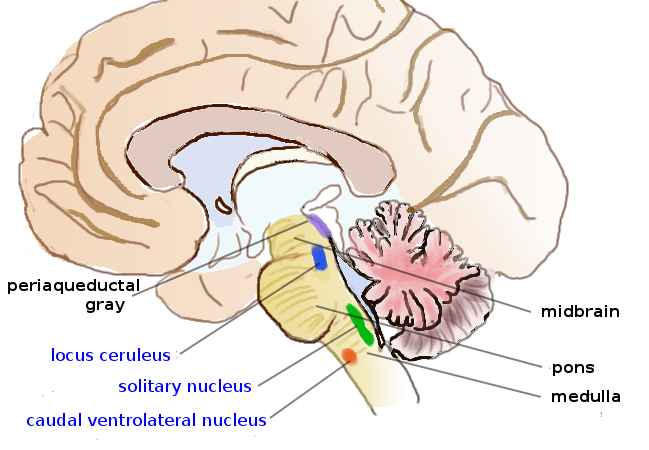
Norepinephrine
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic compound, organic chemical in the catecholamine family that functions in the brain and human body, body as a hormone, neurotransmitter and neuromodulator. The name "noradrenaline" (from Latin '':wikt:ad-, ad'', "near", and '':wikt:ren, ren'', "kidney") is more commonly used in the United Kingdom and the rest of the world, whereas "norepinephrine" (from Ancient Greek :wikt:ἐπί, ἐπῐ́ (''epí''), "upon", and :wikt:νεφρός, νεφρός (''nephrós''), "kidney") is usually preferred in the United States. "Norepinephrine" is also the international nonproprietary name given to norepinephrine (drug), the drug. Regardless of which name is used for the substance itself, parts of the body that produce or are affected by it are referred to as noradrenergic. The general function of norepinephrine is to mobilize the brain and body for action. Norepinephrine release is lowest during sleep, rise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noradrenaline Breakdown
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and body as a hormone, neurotransmitter and neuromodulator. The name "noradrenaline" (from Latin '' ad'', "near", and '' ren'', "kidney") is more commonly used in the United Kingdom and the rest of the world, whereas "norepinephrine" (from Ancient Greek ἐπῐ́ (''epí''), "upon", and νεφρός (''nephrós''), "kidney") is usually preferred in the United States. "Norepinephrine" is also the international nonproprietary name given to the drug. Regardless of which name is used for the substance itself, parts of the body that produce or are affected by it are referred to as noradrenergic. The general function of norepinephrine is to mobilize the brain and body for action. Norepinephrine release is lowest during sleep, rises during wakefulness, and reaches much higher levels during situations of stress or danger, in the so-c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norepinephrine
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic compound, organic chemical in the catecholamine family that functions in the brain and human body, body as a hormone, neurotransmitter and neuromodulator. The name "noradrenaline" (from Latin '':wikt:ad-, ad'', "near", and '':wikt:ren, ren'', "kidney") is more commonly used in the United Kingdom and the rest of the world, whereas "norepinephrine" (from Ancient Greek :wikt:ἐπί, ἐπῐ́ (''epí''), "upon", and :wikt:νεφρός, νεφρός (''nephrós''), "kidney") is usually preferred in the United States. "Norepinephrine" is also the international nonproprietary name given to norepinephrine (drug), the drug. Regardless of which name is used for the substance itself, parts of the body that produce or are affected by it are referred to as noradrenergic. The general function of norepinephrine is to mobilize the brain and body for action. Norepinephrine release is lowest during sleep, rise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-methoxytyramine
3-Methoxytyramine (3-MT), also known as 3-methoxy-4-hydroxyphenethylamine, is a human trace amine and the major metabolite of the monoamine neurotransmitter dopamine. It is formed by the introduction of a methyl group to dopamine by the enzyme catechol-''O''-methyltransferase (COMT). 3-MT can be further metabolized by the enzyme monoamine oxidase (MAO) to form homovanillic acid (HVA), which is then typically excreted in the urine. Occurrence 3-Methoxytyramine occurs naturally in the prickly pear cactus (genus ''Opuntia''), and is in general widespread throughout the Cactaceae. It has also been found in crown gall tumors on ''Nicotiana'' sp. In humans, 3-methoxytyramine is a trace amine that occurs as a metabolite of dopamine. Biological activity Originally thought to be physiologically inactive, 3-MT was subsequently found to act as an agonist of the rodent and human TAAR1. 3-MT can induce weak hyperlocomotion in mice and this effect is partially attenuated in TAAR1 kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanillylmandelic Acid
Vanillylmandelic acid (VMA) is a chemical intermediate in the synthesis of artificial vanilla flavorings and is an end-stage metabolite of the catecholamines (epinephrine, and norepinephrine). It is produced via intermediary metabolites. Chemical synthesis VMA synthesis is the first step of a two-step process practiced by Rhodia since the 1970s to synthesize artificial vanilla. Specifically the reaction entails the condensation of guaiacol and glyoxylic acid in an ice cold, aqueous solution with sodium hydroxide. Biological elimination VMA is found in the urine, along with other catecholamine metabolites, including homovanillic acid (HVA), metanephrine, and normetanephrine. In timed urine tests the quantity excreted (usually per 24 hours) is assessed along with creatinine clearance, and the quantity of cortisols, catecholamines, and metanephrines excreted is also measured. Clinical significance Urinary VMA is elevated in patients with tumors that secrete catecholamines. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |