|
CHN2
Chimerin 2 (beta-chimaerin) is a protein that in humans is encoded by the ''CHN2'' gene. This gene is a member of the chimerin family and encodes a protein with a phorbol-ester/diacylglycerol-type zinc finger, a Rho-GAP domain and an SH2 domain. This protein has GTPase-activating protein activity that is regulated by phospholipid binding and binding of diacylglycerol (DAG) induces translocation of the protein from the cytosol to the Golgi apparatus The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic Cell (biology), cells. Part of the endomembrane system in the cytoplasm, it protein targeting, packages proteins ... membrane. The protein plays a role in the proliferation and migration of smooth muscle cells. Decreased expression of this gene is associated with high-grade gliomas and breast tumors, and increased expression of this gene is associated with lymphomas. Mutations in this gene have been a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chimerin
Chimerin is a type of nerve tissue protein. Chimerins are a family of non-protein kinase C phorbol ester receptors. They were the first phorbol ester receptors to be discovered within this family. They are represented as a family of four closely bound GAPs1 (GTPase-activating proteins). These small GTPases were once characterized as high affinity intracellular receptors for the second messenger diacylglycerol (DAG) and the phorbol ester tumor promoters. The name stems from their resemblance to the "chimera." Types Types include: * Chimerin 1 * Chimerin 2 There are four known isoforms of the chimerin protein. These include α1, α2, β1, and β2. α1-Chimerin was the first protein to be isolated from the brain. The other domains were discovered through alternative splicing. The α and β isoforms are almost identical, the key difference stems from the SH2 domain The SH2 (Src Homology 2) domain is a structurally conserved protein domain contained within the Src oncoprotein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and non-coding genes. During gene expression (the synthesis of Gene product, RNA or protein from a gene), DNA is first transcription (biology), copied into RNA. RNA can be non-coding RNA, directly functional or be the intermediate protein biosynthesis, template for the synthesis of a protein. The transmission of genes to an organism's offspring, is the basis of the inheritance of phenotypic traits from one generation to the next. These genes make up different DNA sequences, together called a genotype, that is specific to every given individual, within the gene pool of the population (biology), population of a given species. The genotype, along with environmental and developmental factors, ultimately determines the phenotype ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phorbol Esters
Phorbol esters are a class of chemical compounds found in a variety of plants, particularly in the families Euphorbiaceae and Thymelaeaceae. Chemically, they are ester derivatives of the tetracyclic diterpenoid phorbol. Biological activity Protein kinase C (PKC) is a phorbol ester receptor. Phorbol esters can stimulate PKC in a similar way to diglycerides. Phorbol esters are known for their ability to promote tumors. In particular, 12-O-tetradecanoylphorbol-13-acetate (TPA) is used as a biomedical research tool in models of carcinogenesis Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cell (biology), cells are malignant transformation, transformed into cancer cells. The process is characterized by changes at the cellular, G .... Plants that contain phorbol esters are often poisonous. References Diterpenes Carboxylate esters Cyclopentenes Plant toxins {{ester-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
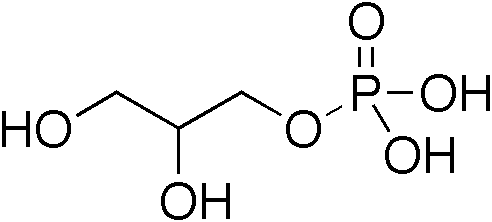
Diacylglycerol
A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. Diglycerides are natural components of food fats, though minor in comparison to triglycerides. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil (particularly 1,3-DAG) has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009. Production Diglycerides are a minor component of many seed oils and are normally present at ~1–6%; or in the case of cottonseed oil as much as 10%. Industrial production is primarily achieved by a glycerolysis reaction between triglycerides and glycerol. The raw materials for this may be either vegetable oil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Finger
A zinc finger is a small protein structural motif that is characterized by the coordination of one or more zinc ions (Zn2+) which stabilizes the fold. The term ''zinc finger'' was originally coined to describe the finger-like appearance of a hypothesized structure from the African clawed frog (''Xenopus laevis'') transcription factor IIIA. However, it has been found to encompass a wide variety of differing protein structures in eukaryotic cells. '' Xenopus laevis'' TFIIIA was originally demonstrated to contain zinc and require the metal for function in 1983, the first such reported zinc requirement for a gene regulatory protein followed soon thereafter by the Krüppel factor in ''Drosophila''. It often appears as a metal-binding domain in multi-domain proteins. Proteins that contain zinc fingers (zinc finger proteins) are classified into several different structural families. Unlike many other clearly defined supersecondary structures such as Greek keys or β hairpins, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GTPase
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases. Functions GTPases function as molecular switches or timers in many fundamental cellular processes. Examples of these roles include: * Signal transduction in response to activation of cell surface receptors, including transmembrane receptors such as those mediating taste, smell and vision. * Protein biosynthesis (a.k.a. translation) at the ribosome. * Regulation of cell differentiation, proliferation, division and movement. * Translocation of proteins through membranes. * Transport of vesicles within the cell, and vesicle-mediated secretion and uptake, through GTPase control of vesicle coat assembly. GTPases are active when bound to GTP and inactive when bound to GDP. In the general ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
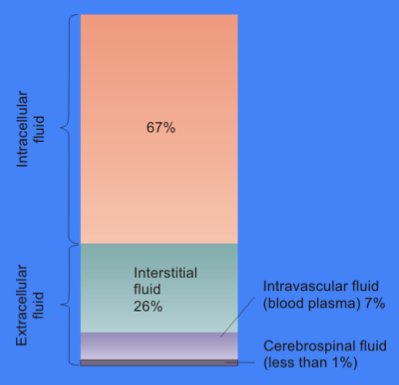
Cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into many compartments. In the eukaryotic cell, the cytosol is surrounded by the cell membrane and is part of the cytoplasm, which also comprises the mitochondria, plastids, and other organelles (but not their internal fluids and structures); the cell nucleus is separate. The cytosol is thus a liquid matrix around the organelles. In prokaryotes, most of the chemical reactions of metabolism take place in the cytosol, while a few take place in membranes or in the periplasmic space. In eukaryotes, while many metabolic pathways still occur in the cytosol, others take place within organelles. The cytosol is a complex mixture of substances dissolved in water. Although water forms the large majority of the cytosol, its structure and proper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
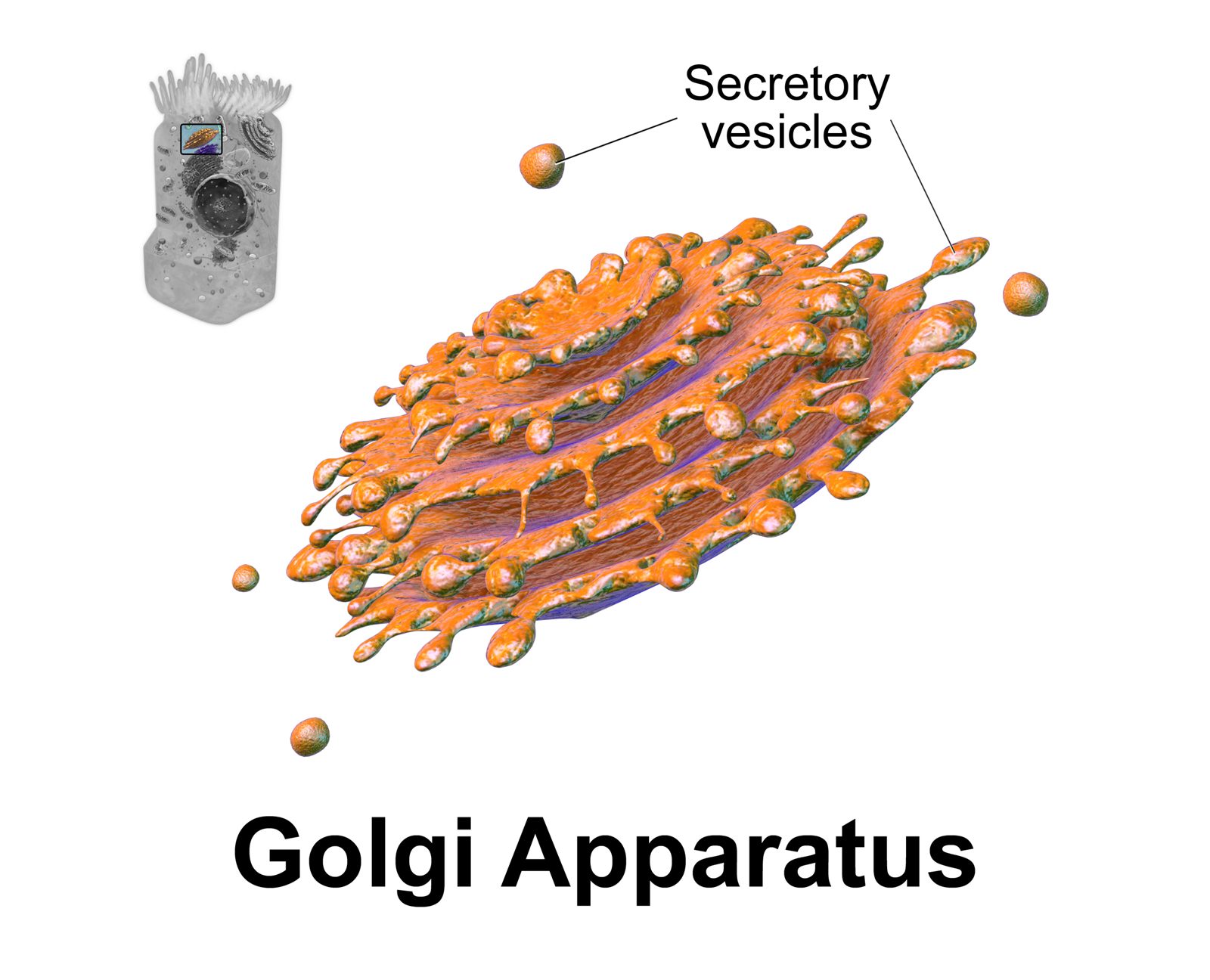
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic Cell (biology), cells. Part of the endomembrane system in the cytoplasm, it protein targeting, packages proteins into membrane-bound Vesicle (biology and chemistry), vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and Endocytosis, endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. The Golgi apparatus was identified in 1898 by the Italian biologist and pathologist Camillo Golgi. The organelle was later named after him in the 1910s. Discovery Because of its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |