|
C10H12O2
The molecular formula C10H12O2 (molar mass : 164.2 g/mol, exact mass: 164.08373 u) may refer to: * Chavibetol * 3,4-Dimethoxystyrene * Duroquinone * Eugenol, a phenylpropene * Isoeugenol, a phenylpropene * Phenethyl acetate * Propyl benzoate * Pseudoisoeugenol * Raspberry ketone * Thujaplicin Thujaplicin (isopropyl cycloheptatrienolone) is any of three isomeric tropolone-related natural products that have been isolated from the softwoods of the trees of ''Cupressaceae'' family. These compounds are known for their antibacterial, antifu ...s ** α-Thujaplicin ** β-Thujaplicin (hinokitiol) ** γ-Thujaplicin * Thymoquinone {{molecular formula index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thujaplicin
Thujaplicin (isopropyl cycloheptatrienolone) is any of three isomeric tropolone-related natural products that have been isolated from the softwoods of the trees of ''Cupressaceae'' family. These compounds are known for their antibacterial, antifungal, and antioxidant properties. They were the first natural tropolones to be made synthetically. History Thujaplicins were discovered in the mid-1930s and purified from the heartwood of ''Thuja plicata'' Donn ex D. Don, commonly called as Western red cedar tree. These compounds were also identified in the constituents of ''Chamaecyparis obtusa'', another species from the ''Cupressaceae'' family. ''C. obtusa'' is native to East Asian countries including Japan and Taiwan, and is also known as ''Taiwan hinoki'', from which the β-thujaplicin was first isolated in 1936 and received its name, ''hinokitiol''. Thujaplicins were the first natural tropolones to be made synthetically, by Ralph Raphael and colleagues, and the β-thujaplicin w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chavibetol
Chavibetol is an organic chemical compound of the phenylpropanoid class. It is one of the primary constituents of the essential oil from the leaves of the betel Betel (''Piper betle'') is a species of flowering plant in the pepper family Piperaceae, native to Southeast Asia. It is an evergreen, dioecious vine, with glossy heart-shaped leaves and white catkins. Betel plants are cultivated for their lea ... plant (''Piper betel'') and catatia. It is an aromatic compound with a spicy odor. Good Scents Company Uses * Decor, candle Chemicals detected in substances or products (note that these chemicals may be absent from an 'ingredient list' for the product and thus unexpected, but have been detected in product testing studies) * Fragrance Fragrances or odor agents, can be used in ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Duroquinone
Duroquinone is an organic Oxidizing agent, oxidant (C6(CH3)4O2). It is related to 1,4-benzoquinone by replacement of four H centres with methyl (Me) groups. The C10O2 core of this molecule is planar with two pairs of C=O and C=C bonds.J.-M. Lü, S. V. Rosokha, I. S. Neretin and J. K. Kochi, "Quinones as Electron Acceptors. X-Ray Structures, Spectral (EPR, UV-vis) Characteristics and Electron-Transfer Reactivities of Their Reduced Anion Radicals as Separated vs Contact Ion Pairs" Journal of the American Chemical Society 2006 128, 16708-16719. The compound is produced via nitration of durene (1,2,4,5-tetramethylbenzene) followed reduction to the diamine and then oxidation. A derived organoiron compound (η2,η2-C6(CH3)4O2)Fe(CO)3 is obtained by the carbonylation of 2-butyne in the presence of iron pentacarbonyl. The molecule has been mentioned in the popular press as a component of a "nano brain". Duroquinone was observed in a degradation products generated from pyrolysis of α-T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eugenol
Eugenol is an allyl chain-substituted guaiacol, a member of the allylbenzene class of chemical compounds. It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove, nutmeg, cinnamon, basil and bay leaf. It is present in concentrations of 80–90% in clove bud oil and at 82–88% in clove leaf oil. Eugenol has a pleasant, spicy, clove-like scent. The name is derived from ''Eugenia caryophyllata'', the former Linnean nomenclature term for cloves. The currently accepted name is ''Syzygium aromaticum''. Biosynthesis The biosynthesis of eugenol begins with the amino acid tyrosine. L-tyrosine is converted to ''p''-coumaric acid by the enzyme tyrosine ammonia lyase (TAL). From here, ''p''-coumaric acid is converted to caffeic acid by ''p''-coumarate 3-hydroxylase using oxygen and NADPH. ''S''-Adenosyl methionine (SAM) is then used to methylate caffeic acid, forming ferulic acid, which is in turn converted to feruloyl- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoeugenol
Isoeugenol is a propenyl-substituted guaiacol. A phenylpropanoid, it occurs in the essential oils of plants such as ylang-ylang (''Cananga odorata''), and is a component of wood smoke and liquid smoke. It can be synthesized from eugenol and has been used in the manufacture of vanillin. It may occur as either the cis ''(Z)'' or trans ''(E)'' isomer In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi .... Trans ''(E)'' isoeugenol is crystalline while cis ''(Z)'' isoeugenol is a liquid. Isoeugenol is one of several phenolic compounds responsible for the mold-inhibiting effect of smoke on meats and cheeses. Allergy Some individuals experience a hives-like reaction to long-term exposure to Isoeugenol, which is named as ''Fragrance'' in the ingredients of consumer products such as soaps, sham ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propyl Benzoate
Propyl benzoate is an organic chemical compound used as a food additive. It is an ester. Uses Propyl benzoate has a nutty odor and sweet fruity or nut-like taste, and as such, it is used as a synthetic flavoring agent in foods. It also has antimicrobial properties and is used as a preservative in cosmetics. It occurs naturally in the sweet cherry and in clove stems, as well as in butter. Reactions Propyl benzoate can be synthesized by the transesterification of methyl benzoate with propanol. Propyl benzoate can also be synthesized by means of Fischer esterification of benzoic acid with propanol There are two isomers of propanol. * 1-Propanol, ''n''-propanol, or propan-1-ol: CH3CH2CH2OH, the most common meaning *2-Propanol, isopropyl alcohol, isopropanol, or propan-2-ol: (CH3)2CHOH See also * Propanal (propionaldehyde) differs in spel .... References Food additives Preservatives Benzoate esters {{Esters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
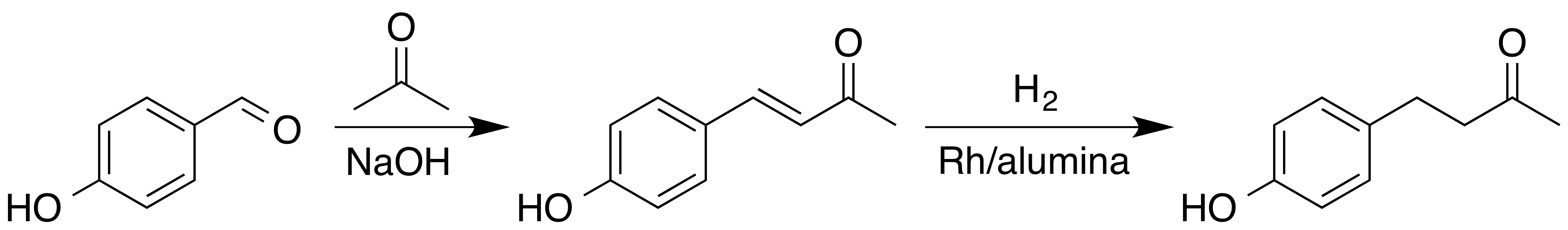
Raspberry Ketone
Raspberry ketone is a naturally occurring phenolic compound that is the primary aroma compound of red raspberry, red raspberries. Occurrence Raspberry ketone occurs in a variety of fruits, including raspberries, cranberry, cranberries, and blackberry, blackberries. It is detected and released by orchid flowers, e.g. ''Dendrobium superbum'' (syn ''D. anosmum''), and several ''Bulbophyllum'' species to attract raspberry ketone-responsive male Dacini fruit flies. It is biosynthesis, biosynthesized from coumaroyl-CoA. It can be extracted from the fruit, yielding about 1–4 mg per kg of raspberries. Preparation Since the natural abundance of raspberry ketone is very low, it is prepared industrially by a variety of methods from chemical intermediates. One of the ways this can be done is through a Claisen-Schmidt condensation followed by catalytic hydrogenation. First, acetone is condensed with 4-hydroxybenzaldehyde to form an Alpha-beta Unsaturated carbonyl compounds, α,β-unsatu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenethyl Acetate
Phenethyl acetate is the ester resulting from the condensation of acetic acid and phenethyl alcohol Phenethyl alcohol, or 2-phenylethanol, is an organic compound with the chemical formula . It is a colourless liquid with a pleasant floral odor. It occurs widely in nature, being found in a variety of essential oils. It is slightly soluble in wate .... Like many esters, it is found in a range of fruits and biological products. It is a colorless liquid with a rose and honey scent and a raspberry-like taste. References Acetate esters {{ester-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudoisoeugenol
Pseudoisoeugenol is a naturally occurring phenylpropene and an isomer of eugenol. Natural occurrence and derivatives Pseudoisoeugenol naturally occurs in the essential oils of roots from plants within the genus ''Pimpinella''. In addition to its standard form, the compound also occurs in a variety of structural derivatives. Common derivatives include the compound with its side chain bearing an epoxide functional group and the aromatic ring being associated with one of many possible esters in the 2nd position. Common esters of the phenol group include angelic acid, 2-methylbutanoic acid, tiglic acid, and 2-methylpropionic acid esters. Hydrolysis of these esters, either ''in vivo'' or by using strong acids, forms 2-methyl-5-methoxybenzofuran. Biosynthesis Biosynthesis of the compound is hypothesized to proceed via a NIH shift of anethole Anethole (also known as anise camphor) is an organic compound that is widely used as a flavoring substance. It is a derivative of the aroma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β-Thujaplicin
Hinokitiol (β-thujaplicin) is a natural monoterpenoid found in the wood of trees in the family Cupressaceae. It is a tropolone derivative and one of the thujaplicins. Hinokitiol is used in oral and skin care products, and is a food additive used in Japan. History Hinokitiol was discovered by a Japanese chemist Tetsuo Nozoe in 1936. It was isolated from the essential oil component of the heartwood of '' Taiwanese hinoki'', from which the compound ultimately adopted its name. Hinokitiol is the first non-benzenoid aromatic compound identified. The compound has a heptagonal molecular structure and was first synthesized by Ralph Raphael in 1951. Due to its iron-chelating activity, hinokitiol has been called an "Iron Man molecule" in the scientific media, which is ironic because Tetsuo is translated into English as "Iron Man". Taiwanese hinoki is native to East Asian countries, particularly to Japan and Taiwan. Hinokitiol has also been found in other trees of the ''Cupressaceae'' fami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |