|
Boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovas and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is found in smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
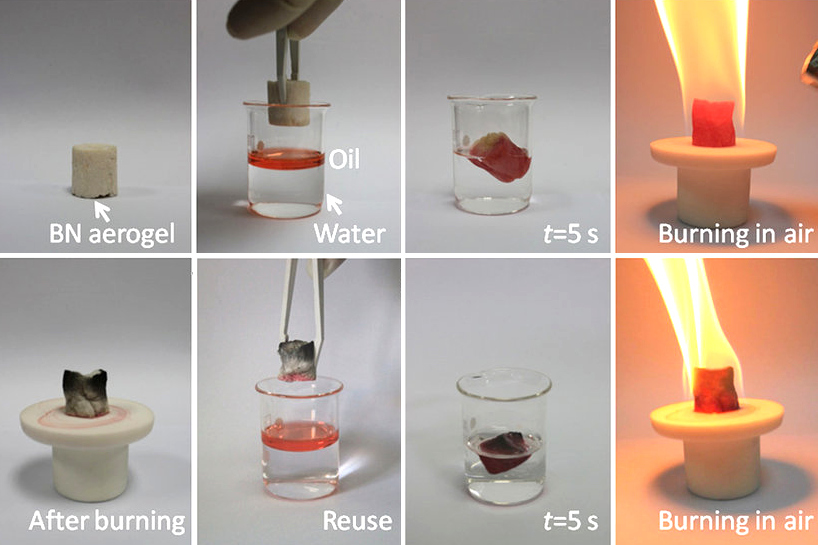
Boron Nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula B N. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. History Boron nitride was discovered by chemistry teacher of the Liverpool Institute in 1842 via reduction of boric acid with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloid
A metalloid is a chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and Nonmetal (chemistry), nonmetals. The word metalloid comes from the Latin language, Latin ''metallum'' ("metal") and the Greek language, Greek ''oeides'' ("resembling in form or appearance"). There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature. The six commonly recognised metalloids are boron, silicon, germanium, arsenic, antimony and tellurium. Five elements are less frequently so classified: carbon, aluminium, selenium, polonium and astatine. On a standard periodic table, all eleven elements are in a diagonal region of the p-block extending from boron at the upper left to astatine at lower right. Some periodic tables include a dividing line between metals and nonmetals, and the metalloids may be found cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Carbide
Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders, as well as numerous industrial applications. With a Vickers hardness of >30 GPa, it is one of the hardest known materials, behind cubic boron nitride and diamond. History Boron carbide was discovered in the 19th century as a by-product of reactions involving metal borides, but its chemical formula was unknown. It was not until the 1930s that the chemical composition was estimated as B4C. Controversy remained as to whether or not the material had this exact 4:1 stoichiometry, as, in practice the material is always slightly carbon-deficient with regard to this formula, and X-ray crystallography shows that its structure is highly complex, with a mixture of C-B-C chains and B12 icosahedra. These features argued against a very simple exact B4C empirical formula. Because of the B12 structural unit, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Group
The boron group are the chemical elements in periodic table group, group 13 of the periodic table, consisting of boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl) and nihonium (Nh). This group lies in the p-block of the periodic table. The elements in the boron group are characterized by having three valence electrons. These elements have also been referred to as the triels. Several group 13 elements have biological roles in the ecosystem. Boron is a trace element in humans and is essential for some plants. Lack of boron can lead to stunted plant growth, while an excess can also cause harm by inhibiting growth. Aluminium has neither a biological role nor significant toxicity and is considered safe. Indium and gallium can stimulate metabolism; gallium is credited with the ability to bind itself to iron proteins. Thallium is highly toxic, interfering with the function of numerous vital enzymes, and has seen use as a pesticide. Characteristics Like other groups, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Boron
Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of its α-tetragonal (α-T) and γ-orthorhombic (γ) allotropes. Two amorphous forms, one a finely divided powder and the other a glassy solid, are also known. Although at least 14 more allotropes have been reported, these other forms are based on tenuous evidence or have not been experimentally confirmed, or are thought to represent mixed allotropes, or boron frameworks stabilized by impurities. Whereas the β-rhombohedral phase is the most stable and the others are metastable, the transformation rate is negligible at room temperature, and thus all five phases can exist at ambient conditions. Amorphous powder boron and polycrystalline β-rhombohedral boron are the most common forms. The latter allotrope is a very hard Vickers hardness compar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boric Acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salt (chemistry), salts, and can react with Alcohol (chemistry), alcohols to form borate esters. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other boron compounds. The term "boric acid" is also used generically for any oxyacid of boron, such as metaboric acid and tetraboric acid . History Orthoboric acid was first prepared by Wilhelm Homberg (1652–1715) from borax, by the action of mineral acids, and was given the name ("sedative salt of Homberg"). However, boric acid and borates have been used since the time of the ancient Greece, anc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Fiber
Boron fiber or boron filament is an amorphous product which represents the major industrial use of elemental boron. Boron fiber manifests a combination of high strength and high elastic modulus. A common use of boron fibers is in the construction of high tensile strength tapes. Boron fiber use results in high-strength, lightweight materials that are used chiefly for advanced aerospace structures as a component of composite materials, as well as limited production consumer and sporting goods such as golf clubs and fishing rods. One of the uses of boron fiber composites was the horizontal tail surfaces of the F-14 Tomcat fighter. This was done because carbon fiber composites were not then developed to the point they could be used, as they were in many subsequent aircraft designs. Boron fiber is a primary reinforcement constituent in Hy-Bor, a prepreg blend of boron fiber and carbon fiber primarily used for high-performance aerospace and sporting goods applications. In the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Symbol
Chemical symbols are the abbreviations used in chemistry, mainly for chemical elements; but also for functional groups, chemical compounds, and other entities. Element symbols for chemical elements, also known as atomic symbols, normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek language, Greek words. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (element), mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a Neo-Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter Systematic element name, temporary sym ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borosilicate Glass
Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. Borosilicate glasses are known for having very low coefficients of thermal expansion (≈3 × 10−6 K−1 at 20 °C), making them more resistant to thermal shock than any other common glass. Such glass is subjected to less thermal stress and can withstand temperature differentials without fracturing of about . It is commonly used for the construction of reagent bottles and flasks, as well as lighting, electronics, and cookware. For many other applications, soda-lime glass is more common. Borosilicate glass is sold under various trade names, including Borosil, Duran, Pyrex, Glassco, Supertek, Suprax, Simax, Bellco, Marinex (Brazil), BSA 60, BSC 51 (by NIPRO), Heatex, Endural, Schott, Refmex, Kimax, Gemstone Well, United Scientific, and MG (India). Single-ended self-starting lamps are insulated with a mica disc and contained in a borosilicate glass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kernite
Kernite, also known as rasorite, is a hydrated sodium borate hydroxide mineral with formula . It is a colorless to white mineral crystallizing in the monoclinic crystal system typically occurring as prismatic to acicular (crystal habit), acicular crystals or granular masses. It is relatively soft with Mohs hardness of 2.5 to 3 and light with a specific gravity of 1.91. It exhibits perfect cleavage and a brittle fracture. Kernite is soluble in cold water and alters to tincalconite when it dehydrates. It undergoes a non-reversible alteration to metakernite () when heated to above 100 °C. Occurrence and history The mineral occurs in sedimentary evaporite deposits in arid regions. Kernite was discovered in 1926 in eastern Kern County, California, Kern County, in Southern California, and later renamed after the county. The location was the Pacific Coast Borax Company#U.S. Borax, US Borax Mine at Boron, California, Boron in the western Mojave Desert. This Type specimen (mineralog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cosmic Ray Spallation
Cosmic ray spallation, also known as the x-process, is a set of naturally occurring nuclear reactions causing nucleosynthesis; it refers to the formation of chemical elements from the impact of cosmic rays on an object. Cosmic rays are highly energetic charged particles from beyond Earth, ranging from protons, alpha particles, and nuclei of many heavier elements. About 1% of cosmic rays also consist of free electrons. Cosmic rays cause spallation when a ray particle (e.g. a proton) impacts with matter, including other cosmic rays. The result of the collision is the expulsion of particles (protons, neutrons, and alpha particles) from the object hit. This process goes on not only in deep space, but in Earth's upper atmosphere and crustal surface (typically the upper ten meters) due to the ongoing impact of cosmic rays. The process Cosmic ray spallation is thought to be responsible for the abundance in the universe of some light elements—lithium, beryllium, and boron—as well ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical properties, such as its valence—whether it may bond with other elements and, if so, how readily and with how many. In this way, a given element's reactivity is highly dependent upon its electronic configuration. For a main-group element, a valence electron can exist only in the outermost electron shell; for a transition metal, a valence electron can also be in an inner shell. An atom with a closed shell of valence electrons (corresponding to a noble gas configuration) tends to be chemically inert. Atoms with one or two valence electrons more than a closed shell are highly reactive due to the relativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |