|
Benzaldehydes
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful. It is a colorless liquid with a characteristic almond-like odor, and is commonly used in cherry-flavored sodas. A component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources. Synthetic benzaldehyde is the flavoring agent in imitation almond extract, which is used to flavor cakes and other baked goods. History Benzaldehyde was first extracted in 1803 by the French pharmacist Martrès. His experiments focused on elucidating the nature of amygdalin, the poisonous compound found in bitter almonds, the fruit of ''Prunus dulcis''. Further work on the oil by Pierre Robiquet and Antoine Boutron Charlard, two French chemists, produced benzaldehyde. In 1832, Friedrich Wöhler and Justus von Liebig first synthesized benzaldehyde. Production Benzaldehyde can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prunus Dulcis
The almond (''Prunus amygdalus'', syn. ''Prunus dulcis'') is a species of tree from the genus ''Prunus''. Along with the peach, it is classified in the subgenus ''Amygdalus'', distinguished from the other subgenera by corrugations on the shell (endocarp) surrounding the seed. The fruit of the almond is a drupe, consisting of an outer hull and a hard shell with the seed, which is not a true nut. ''Shelling'' almonds refers to removing the shell to reveal the seed. Almonds are sold shelled or unshelled. Blanched almonds are shelled almonds that have been treated with hot water to soften the seedcoat, which is then removed to reveal the white embryo. Once almonds are cleaned and processed, they can be stored for around a year if kept refrigerated; at higher temperatures they will become rancid more quickly. Almonds are used in many cuisines, often featuring prominently in desserts, such as marzipan. The almond tree prospers in a moderate Mediterranean climate with cool win ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
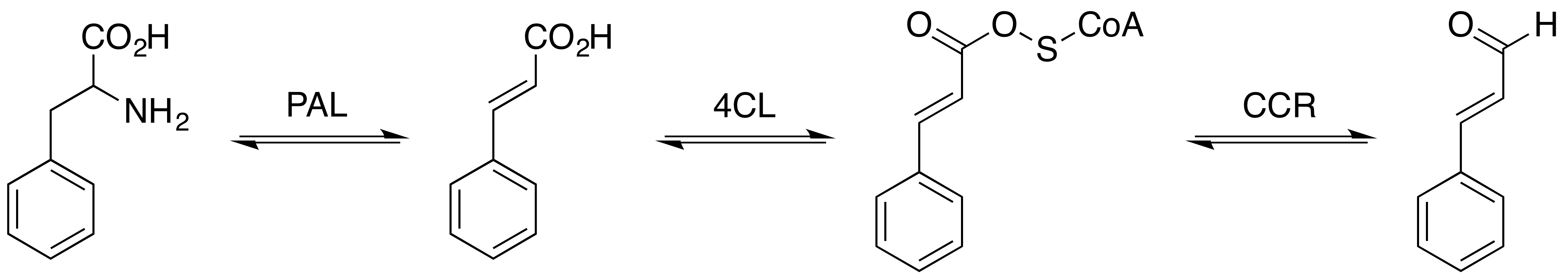
Cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula or . Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus '' Cinnamomum''. It is an essential oil. The bark of cinnamon tree contains high concentrations of cinnamaldehyde. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is '' trans''-cinnamaldehyde. The molecule consists of a benzene ring attached to an unsaturated aldehyde. Cinnamaldehyde is an α,β-unsaturated carbonyl compound. Its color is due to the π → π* transition: increased conjugation in comparison with acrolein shifts this band towa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
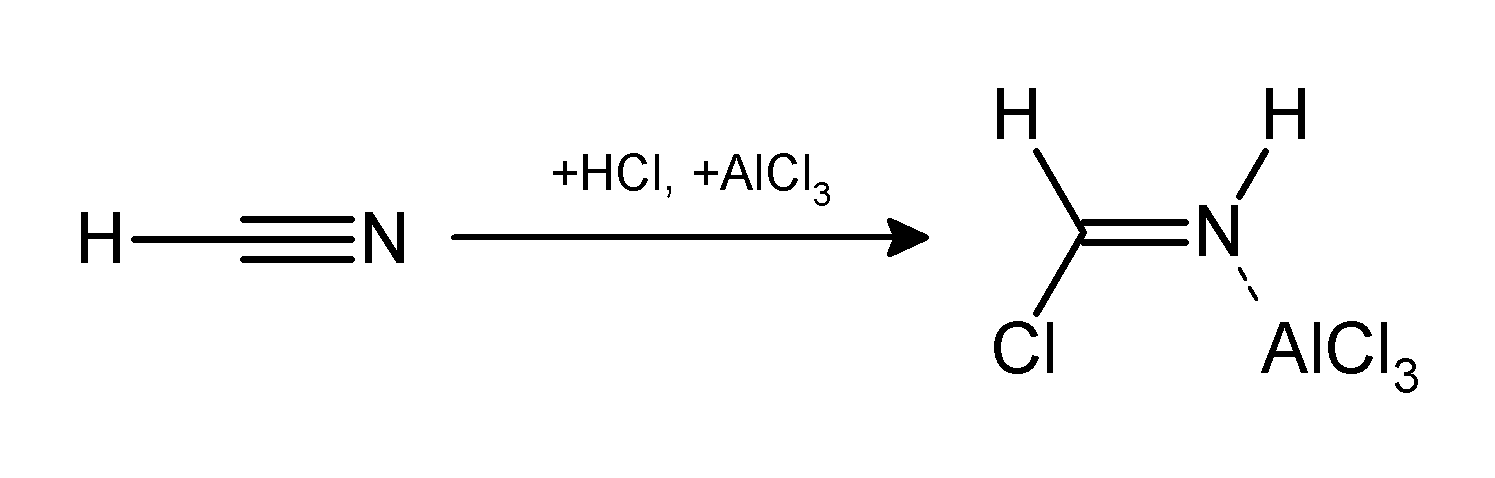
Gatterman-Koch Reaction
The Gattermann reaction (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as aluminium chloride (AlCl3). It is named for the German chemist Ludwig Gattermann and is similar to the Friedel–Crafts reaction. Modifications have shown that it is possible to use sodium cyanide or cyanogen bromide in place of hydrogen cyanide. The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide. Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN. The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(Cl)2 that serves as the Lewis-acid catalyst ''in-situ''. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene. Gattermann–Koch reaction The Gattermann– ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonylation
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbonylation also refers to oxidation of protein side chains. Organic chemistry Several industrially useful organic chemicals are prepared by carbonylations, which can be highly selective reactions. Carbonylations produce organic carbonyls, i.e., compounds that contain the functional group such as aldehydes (), carboxylic acids () and esters (). Carbonylations are the basis of many types of reactions, including hydroformylation and Reppe reactions. These reactions require metal catalysts, which bind and activate the CO. These processes involve transition metal acyl complexes as intermediates. Much of this theme was developed by Walter Reppe. Hydroformylation Hydroformylation entails the addition of both carbon monoxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzal Chloride
Benzal chloride is an organic compound with the formula C6H5CHCl2. This colourless liquid is a lachrymator and is used as a building block in organic synthesis. Preparation and usage Benzal chloride is produced by the free radical chlorination of toluene, being preceded in the process by benzyl chloride (C6H5CH2Cl) and followed by benzotrichloride (C6H5CCl3): : C6H5CH3 + Cl2 → C6H5CH2Cl + HCl : C6H5CH2Cl + Cl2 → C6H5CHCl2 + HCl : C6H5CHCl2 + Cl2 → C6H5CCl3 + HCl Benzylic halides are typically strong alkylating agents, and for this reason benzal chloride is treated as a hazardous compound. Treatment of benzal chloride with sodium gives stilbene. Most benzal chloride main industrial use is as a precursor to benzaldehyde. This conversion involves hydrolysis Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases. Etymology The word ''alkali'' is derived from Arabic ''al qalīy'' (or ''alkali''), meaning (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (''slaked lime''), a far more strongly basic substance known as ''caustic potash ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl Alcohol
Benzyl alcohol (also known as α-cresol) is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is useful as a solvent for its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide. Natural occurrences Benzyl alcohol is produced naturally by many plants and is commonly found in fruits and teas. It is also found in a variety of essential oils including jasmine, hyacinth and ylang-ylang. It is also found in castoreum from the castor sacs of beavers. Benzyl esters also occur naturally. Preparation Benzyl alcohol is produced industrially from toluene via ben ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Oxidation
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen.''Organic Redox Systems: Synthesis, Properties, and Applications'', Tohru Nishinaga 2016 Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation: When methane is oxidized to carbon dioxide its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include alcohol oxidation, oxidation of alcohols to aldehydes. In oxidation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...-insoluble liquid with the odor associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group by a single bond. As such, its systematic IUPAC nomenclature of organic chemistry, IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jones Oxidation
The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent. Jones reagent is a solution prepared by dissolving chromium trioxide in aqueous sulfuric acid. To effect a Jones oxidation, this acidic mixture is then added to an acetone solution of the substrate. Alternatively, potassium dichromate can be used in place of chromium trioxide. The oxidation is very rapid and quite exothermic. Yields are typically high. The reagent is convenient and cheap. However, Cr(VI) compounds are carcinogenic, which deters the use of this methodology. Stoichiometry and mechanism Jones reagent will convert primary and secondary alcohols to aldehydes and ketones, respectively. Depending on the reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |