Jones Oxidation on:
[Wikipedia]
[Google]
[Amazon]
The Jones oxidation is an  Jones reagent is a solution prepared by dissolving
Jones reagent is a solution prepared by dissolving
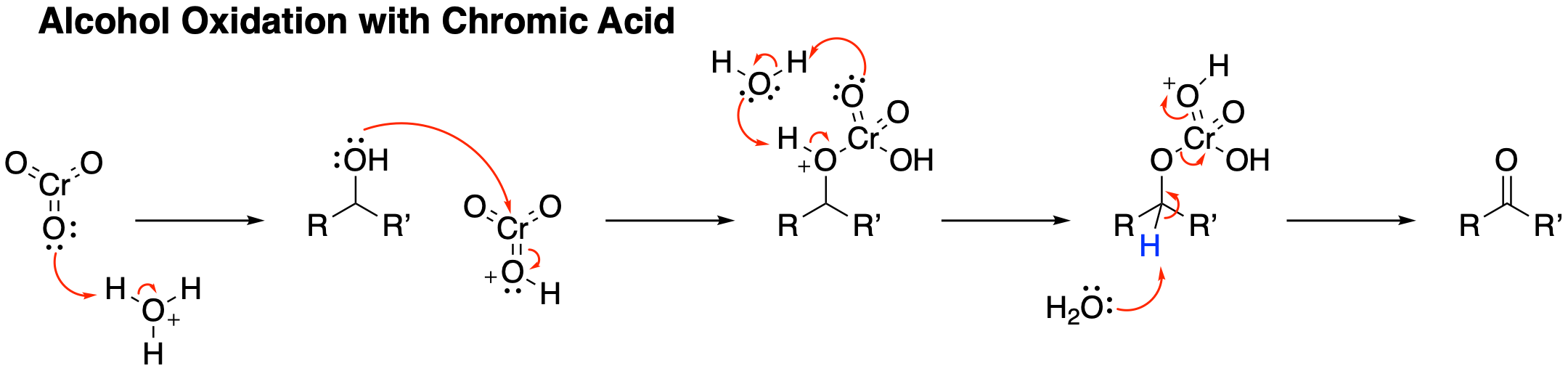 The reaction stoichiometry implicates the Cr(IV) species "CrO2OH−", which comproportionates with the chromic acid to give a Cr(V) oxide, which also functions as an oxidant for the alcohol.
The oxidation of the aldehydes is proposed to proceed via the formation of hemiacetal-like intermediates, which arise from the addition of the O3CrO-H− bond across the C=O bond.
The reagent rarely oxidizes unsaturated bonds.
The reaction stoichiometry implicates the Cr(IV) species "CrO2OH−", which comproportionates with the chromic acid to give a Cr(V) oxide, which also functions as an oxidant for the alcohol.
The oxidation of the aldehydes is proposed to proceed via the formation of hemiacetal-like intermediates, which arise from the addition of the O3CrO-H− bond across the C=O bond.
The reagent rarely oxidizes unsaturated bonds.
organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical rea ...
for the oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of primary and secondary alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s to carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
s and ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
s, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
 Jones reagent is a solution prepared by dissolving
Jones reagent is a solution prepared by dissolving chromium trioxide
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.
This compound is a dark-purple ...
in aqueous sulfuric acid. To effect a Jones oxidation, this acidic mixture is then added to an acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscible wi ...
solution of the substrate. Alternatively, potassium dichromate
Potassium dichromate, , is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to healt ...
can be used in place of chromium trioxide. The oxidation is very rapid and quite exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity ...
. Yields are typically high. The reagent is convenient and cheap. However, Cr(VI) compounds are carcinogenic, which deters the use of this methodology.Stoichiometry and mechanism
Jones reagent will convert primary and secondary alcohols to aldehydes and ketones, respectively. Depending on the reaction conditions, the aldehydes may then be converted to carboxylic acids. For oxidations to the aldehydes and ketones, two equivalents of chromic acid oxidize three equivalents of the alcohol: : 2 HCrO4− + 3 RR'C(OH)H + 8 H+ + 4 H2O → 2 r(H2O)6sup>3+ + 3 RR'CO For oxidation of primary alcohols to carboxylic acids, 4 equivalents of chromic acid oxidize 3 equivalents of the alcohol. The aldehyde is an intermediate. :4 HCrO4− + 3 RCH2OH + 16 H+ + 11 H2O → 4 r(H2O)6sup>3+ + 3 RCOOH The inorganic products are green, characteristic of chromium(III) aquo complexes. Like many other oxidations of alcohols by metal oxides, the reaction proceeds via the formation of a mixed chromate ester: Theseester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
s have the formula CrO3(OCH2R)−
:CrO3(OH)− + RCH2OH → CrO3(OCH2R)− + H2O
Like conventional esters, the formation of this chromate ester is accelerated by the acid. These esters can be isolated when the alcohol is tertiary because these lack the α hydrogen that would be lost to form the carbonyl. For example, using ''tert''-butyl alcohol, one can isolate ''tert''-butyl chromate ((CH3)3CO)2CrO2), which is itself a good oxidant.
For those structures with hydrogen alpha to the oxygen, the chromate esters degrade, releasing the carbonyl product and an ill-defined Cr(IV) product:
:CrO3(OCH2R)− → CrO2OH− + O=CHR
The deuterated alcohols HOCD2R oxidize about six times slower than the undeuterated derivatives. This large kinetic isotope effect shows that the C–H (or C–D) bond breaks in the rate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
.
 The reaction stoichiometry implicates the Cr(IV) species "CrO2OH−", which comproportionates with the chromic acid to give a Cr(V) oxide, which also functions as an oxidant for the alcohol.
The oxidation of the aldehydes is proposed to proceed via the formation of hemiacetal-like intermediates, which arise from the addition of the O3CrO-H− bond across the C=O bond.
The reagent rarely oxidizes unsaturated bonds.
The reaction stoichiometry implicates the Cr(IV) species "CrO2OH−", which comproportionates with the chromic acid to give a Cr(V) oxide, which also functions as an oxidant for the alcohol.
The oxidation of the aldehydes is proposed to proceed via the formation of hemiacetal-like intermediates, which arise from the addition of the O3CrO-H− bond across the C=O bond.
The reagent rarely oxidizes unsaturated bonds.
Illustrative reactions and applications
It remains useful inorganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. A variety of spectroscopic techniques, including Infrared spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
, can be used to monitor the progress of a Jones oxidation reaction. At one time the Jones oxidation was used in breathalyzer
A breathalyzer or breathalyser (a portmanteau of ''breath'' and ''analyzer/analyser'') is a device for estimating blood alcohol content (BAC), or to detect viruses or diseases from a breath sample.
The name is a genericized trademark of the Br ...
s.
Related processes
The principal reagents are Collins reagent, PDC, and PCC. These reagents represent improvements over inorganic chromium(VI) reagents such as Jones reagent.Historical references
* * * * * * *References
{{reflist Organic oxidation reactions Name reactions