|
Amidines
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2. Examples of amidines include: * DBU * diminazene * benzamidine * Pentamidine * Paranyline Preparation A common route to primary amidines is the Pinner reaction. Reaction of the nitrile with alcohol in the presence of acid gives an iminoether. Treatment of the resulting compound with ammonia then completes the conversion to the amidine. Instead of using a Bronsted acid, Lewis acids such as aluminium trichloride promote the direct amination of nitriles, or, in certain exceptional cases, of amides. Dimethylformamide acetal reacts with primary amines to give amidines: :Me2NC(H)(OMe)2 + RNH2 → Me2NC=NHR + 2 MeOH Catalysis is likewise not required for direct amination of an imidoyl chloride. Amidines are also prepared by the addition of organolithi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetamidine Structural Formulae V
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2. Examples of amidines include: * 1,8-Diazabicycloundec-7-ene, DBU * diminazene * benzamidine * Pentamidine * Paranyline Preparation A common route to primary amidines is the Pinner reaction. Reaction of the nitrile with alcohol in the presence of acid gives an Carboximidate, iminoether. Treatment of the resulting compound with ammonia then completes the conversion to the amidine. Instead of using a Brønsted–Lowry acid–base theory, Bronsted acid, Lewis acids and bases, Lewis acids such as Aluminium chloride, aluminium trichloride promote the direct amination of nitriles, or, in certain exceptional cases, of amides. Dimethylformamide acetal reacts with primary amines to give amidines: :Me2NC(H)(OMe)2 + RNH2 → Me2NC=NHR + 2 MeOH Catalysis is likewise not require ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzamidine
Benzamidine is an organic compound with the formula C6H5C(NH)NH2. It is the simplest aryl amidine. The compound is a white solid that is slightly soluble in water. It is usually handled as the hydrochloride salt, a white, water-soluble solid. Structure Benzamidine has one short C=NH bond and one longer C-NH2 bond, which are respectively 129 and 135 pm in length, respectively. The triangular diamine group gives it a distinctive shape which shows up in difference density maps. Applications Benzamidine is a reversible competitive inhibitor of trypsin, trypsin-like enzymes, and serine proteases. It is often used as a ligand in protein crystallography to prevent proteases from degrading a protein of interest. The benzamidine moiety is also found in some pharmaceuticals, such as dabigatran Dabigatran, sold under the brand name Pradaxa among others, is an anticoagulant used to treat and prevent blood clots and to prevent stroke in people with atrial fibrillation. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amitraz
Amitraz (development code BTS27419) is a non-systemic acaricide and insecticideCorta, E., Bakkali, A., Berrueta, L. A., Gallo, B., & Vicente, F. (1999). Kinetics and mechanism of amitraz hydrolysis in aqueous media by HPLC and GC-MS. Talanta, 48(1), 189-199 and has also been described as a scabicide. It was first synthesized by the Boots Co. in England in 1969.Harrison, I. R., et al. (1973). 1,3,5-Triazapenta-1, 4-dienes: Chemical aspects of a new group of pesticides. Pestic. Sci. 4: 901 Amitraz has been found to have an insect repellent effect, works as an insecticide and also as a pesticide Synergy#Biological_sciences, synergist.PubChem Substance. Amitraz – Substance Summary. retrieved from https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=24868774#x332 Its effectiveness is traced back on Alpha-adrenergic agonist, alpha-adrenergic agonist activity, interaction with octopamine receptors of the central nervous system and inhibition of monoamine oxidases and prostaglandin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
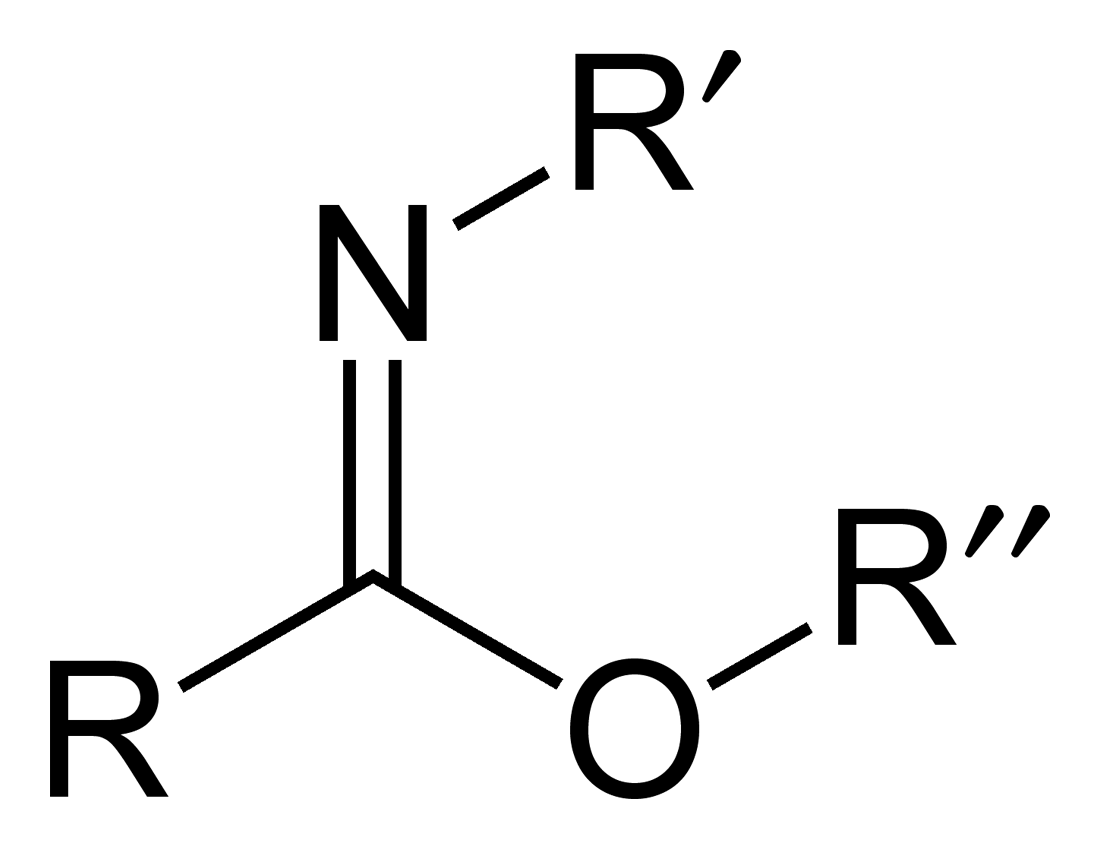
Carboximidate
Carboximidates (or more general imidates) are organic compounds, which can be thought of as esters formed between an imidic acid () and an alcohol, with the general formula . They are also known as imino ethers, since they resemble imines () with an oxygen atom connected to the carbon atom of the C=N double bond. Synthesis Imidates may be generated by a number of synthetic routes, but are in general formed by the Pinner reaction. This proceeds via the acid catalyzed attack of nitriles by alcohols. Imidates produced in this manner are formed as their hydrochloride salts, which are sometimes referred to as Pinner salts. Carboximidates are also formed as intermediates in the Mumm rearrangement and the Overman rearrangement. Imidate/amidate anions An amidate/imidate anion is formed upon deprotonation of an amide or imidic acid. Since amides and imidic acids are tautomers, they form the same anion upon deprotonation. The two names are thus synonyms describing the same anion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentamidine
Pentamidine is an antimicrobial medication used to treat African trypanosomiasis, leishmaniasis, '' Balamuthia'' infections, babesiosis, and to prevent and treat pneumocystis pneumonia (PCP) in people with poor immune function. In African trypanosomiasis it is used for early disease before central nervous system involvement, as a second line option to suramin. It is an option for both visceral leishmaniasis and cutaneous leishmaniasis. Pentamidine can be given by injection into a vein or muscle or by inhalation. Common side effects of the injectable form include low blood sugar, pain at the site of injection, nausea, vomiting, low blood pressure, and kidney problems. Common side effects of the inhaled form include wheezing, cough, and nausea. It is unclear if doses should be changed in those with kidney or liver problems. Pentamidine is not recommended in early pregnancy but may be used in later pregnancy. Its safety during breastfeeding is unclear. Pentamidine is in the arom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xylamidine
Xylamidine is a drug which acts as an antagonist of the serotonin 5-HT2A and 5-HT2C receptors, and to a lesser extent of the serotonin 5-HT1A receptor. The drug does not cross the blood–brain barrier and hence is peripherally selective, which makes it useful for blocking peripheral serotonergic responses like cardiovascular and gastrointestinal effects, without producing the central effects of 5-HT2A receptor blockade such as sedation, or interfering with the central actions of 5-HT2A receptor agonists. Xylamidine and analogues were patented for use in combination with serotonin 5-HT2A receptor agonists like serotonergic psychedelics in 2023. Chemistry Synthesis Xylamidine is an amidine. It is prepared by alkylation of 3-methoxyphenol (''m''-methoxyphenol) with α- chloropropionitrile, potassium iodide, and potassium carbonate in butanone to give #, which is in turn reduced with lithium aluminium hydride to give the primary amine #. When # is treated with ''m''- tolylac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diminazene
Diminazene (INN; also known as diminazen) is an anti-infective medication for animals that is sold under a variety of brand names. It is effective against certain protozoa such as ''Babesia'', ''Trypanosoma'', and '' Cytauxzoon''. The drug may also be effective against certain bacteria including ''Brucella'' and ''Streptococcus''. Chemically it is a di-amidine and it is formulated as its aceturate salt, diminazene aceturate. The mechanism is not well understood; it probably inhibits DNA replication, but also has affinity to RNA. Side effects Acute side effects include vomiting, diarrhea, and hypotension (low blood pressure). Diminazen can harm the liver, kidneys and brain, which is potentially life-threatening; camels are especially susceptible to these effects. Resistance The Gibe River Valley in southwest Ethiopia showed universal resistance between July 1989 and February 1993. This likely indicates a permanent loss of function in this area against the tested target, '' T. c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,8-Diazabicycloundec-7-ene
1,8-Diazabicyclo .4.0ndec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst, a complexing ligand, and a non-nucleophilic base. Synthesis DBU can be synthesised in three steps from caprolactam and acrylonitrile. First, the nitrile undergoes an addition reaction at the caprolactam nitrogen, producing ''N''-(2-cyanoethyl)-caprolactam. This is then hydrogenated to ''N''-aminopropyl-caprolactam, followed by an intramolecular carbonyl condensation to form the imine. Water is removed by fractional distillation. Occurrence Although all commercially available DBU is produced synthetically, it may also be isolated from the sea sponge '' Niphates digitalis''. The biosynthesis of DBU has been proposed to begin with adipaldehyde and 1,3-diaminopropane. Applications DBU is used: * As a reagent in organic chemistry, where DBU is used as a ligand and base. As a base, protonation occurs at the i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insecticide
Insecticides are pesticides used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. The major use of insecticides is in agriculture, but they are also used in home and garden settings, industrial buildings, for vector control, and control of insect parasites of animals and humans. Acaricides, which kill mites and ticks, are not strictly insecticides, but are usually classified together with insecticides. Some insecticides (including common bug sprays) are effective against other non-insect arthropods as well, such as scorpions, spiders, etc. Insecticides are distinct from insect repellents, which repel but do not kill. Sales In 2016 insecticides were estimated to account for 18% of worldwide pesticide sales. Worldwide sales of insecticides in 2018 were estimated as $ 18.4 billion, of which 25% were neonicotinoids, 17% were pyrethroids, 13% were diamides, and the rest were many other classes which sold for less th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidocarb
Imidocarb is a urea derivative used in veterinary medicine as an antiprotozoal agent for the treatment of infection with ''Babesia'' ( babesiosis) and other parasites. Mechanism of action Imidocarb is anticholinergic; it inhibits acetylcholinesterase Acetylcholinesterase (HUGO Gene Nomenclature Committee, HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme th .... References Antiprotozoal agents Ureas Imidazolines {{Antiinfective-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |