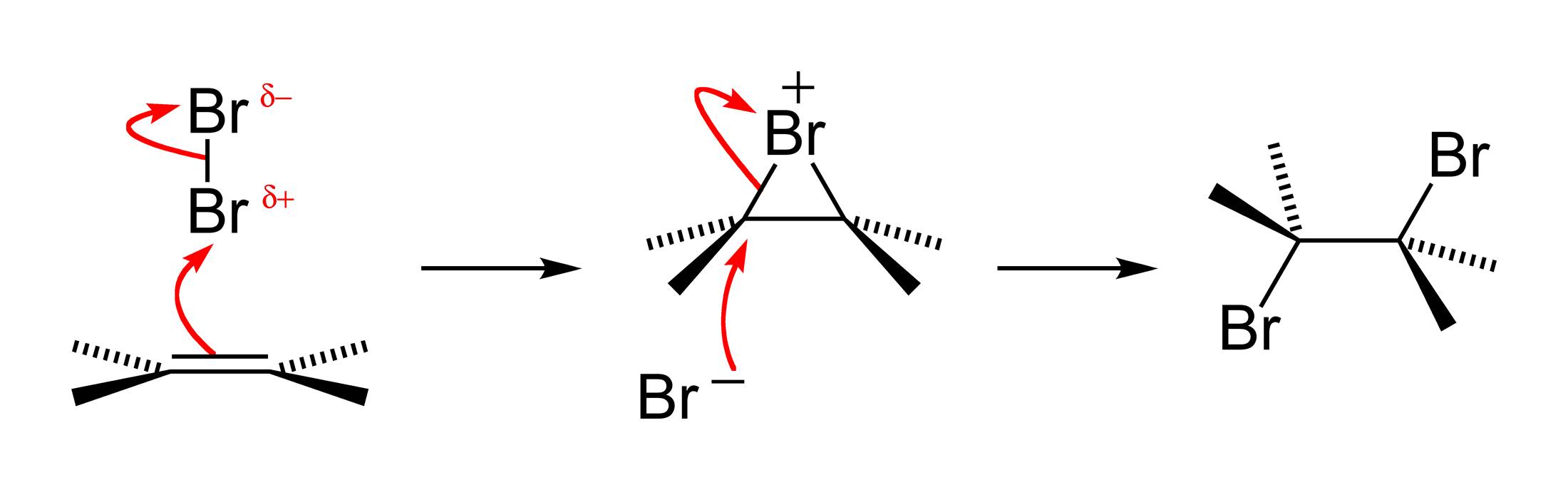|
Bromonium
A halonium ion is any onium ion containing a halogen atom carrying a positive charge. This cation has the general structure where X is any halogen and no restrictions on R, this structure can be cyclic or an open chain molecular structure. Halonium ions formed from fluorine, chlorine, bromine, and iodine are called fluoronium, chloronium, bromonium, and iodonium, respectively. The 3-membered cyclic variety commonly proposed as intermediates in electrophilic halogenation may be called haliranium ions, using the Hantzsch-Widman nomenclature system. Structure The simplest halonium ions are of the structure (X = F, Cl, Br, I). Many halonium ions have a three-atom cyclic structure, similar to that of an epoxide, resulting from the formal addition of a halogenium ion to a C=C double bond, as when a halogen is added to an alkene. The formation of 5-membered halonium ions (e.g., chlorolanium, bromolanium ions) via neighboring group participation is also well studied. Diaryliodoniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen Addition Reaction
A halogen addition reaction is a simple organic reaction where a halogen molecule is added to the carbon–carbon double bond of an alkene functional group. The general chemical formula of the halogen addition reaction is: :C=C + X2 → X−C−C−X (X represents the halogens bromine or chlorine, and in this case, a solvent could be CH2Cl2 or CCl4). The product is a vicinal dihalide. This type of reaction is a halogenation and an electrophilic addition. Reaction mechanism The reaction mechanism for an alkene bromination can be described as follows. In the first step of the reaction, a bromine molecule approaches the electron-rich alkene carbon–carbon double bond. The bromine atom closer to the bond takes on a partial positive charge as its electrons are repelled by the electrons of the double bond. The atom is electrophilic at this time and is attacked by the pi electrons of the alkene arbon–carbon double bond It forms for an instant a single sigma bond to ''both' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. Electrophiles mainly interact with nucleophiles through Addition reaction, addition and Substitution reaction, substitution reactions. Frequently seen electrophiles in Organic synthesis, organic syntheses include cations such as Hydrogen ion, H+ and nitrosonium, NO+, polarized neutral molecules such as hydrogen chloride, HCl, alkyl halides, acyl halides, and carbonyl compounds, polarizable neutral molecules such as chlorine, Cl2 and bromine, Br2, oxidizing agents such as organic peracids, chemical species that do not satisfy the octet rule such as carbenes and Radical (chemistry), radicals, and some Lewis acids such as Borane, BH3 and Di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Onium Ion
In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride of a pnictogen (group 15 of the periodic table), chalcogen (group 16), or halogen (group 17). The oldest-known onium ion, and the namesake for the class, is ammonium, , the protonated derivative of ammonia, . The name onium is also used for cations that would result from the substitution of hydrogen atoms in those ions by other groups, such as organic groups, or halogens; such as tetraphenylphosphonium, . The substituent groups may be divalent or trivalent, yielding ions such as iminium and nitrilium. A simple onium ion has a charge of +1. A larger ion that has two onium ion subgroups is called a double onium ion, and has a charge of +2. A triple onium ion has a charge of +3, and so on. Compounds of an onium cation and some other anion are known as onium compounds or onium salts. Onium ions and onium compounds are inversely analogous to ions and ate complexe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived , referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a free element in nature. Instead, it can be isolated from colourless soluble crystalline mineral halide Ionic salt, salts analogous to table salt, a property it shares with the other halogens. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its Bromine cycle, accumulation in the oceans. Commercially the element is easily extracted from brine evaporation ponds, mostly in the United States and Israel. The mass of bromine in the oce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Properties Of Water
Water () is a Chemical polarity, polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from Color of water, an inherent hint of blue. It is by far the most studied chemical compound and is described as the "universal solvent" and the "solvent of life". It is the most abundant substance on the surface of Earth and the only common substance to exist as a ice, solid, liquid, and water vapor, gas on Earth's surface. It is also the third most abundant molecule in the universe (behind Hydrogen, molecular hydrogen and carbon monoxide). Water molecules form hydrogen bonds with each other and are strongly polar. This polarity allows it to dissociate ions in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form, a relatively high boiling point of 100 °C for its molar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halohydrin
In organic chemistry a halohydrin (also a haloalcohol or β-halo alcohol) is a functional group in which a halogen and a hydroxyl are bonded to adjacent carbon atoms, which otherwise bear only hydrogen or hydrocarbyl groups (e.g. 2-chloroethanol, 3-chloropropane-1,2-diol). The term only applies to saturated motifs, as such compounds like 2-chlorophenol would not normally be considered halohydrins. Megatons of some chlorohydrins, e.g. propylene chlorohydrin, are produced annually as precursors to polymers. Halohydrins may be categorized as chlorohydrins, bromohydrins, fluorohydrins or iodohydrins depending on the halogen present. Synthesis From alkenes Halohydrins are usually prepared by treatment of an alkene with a halogen, in the presence of water. The reaction is a form of electrophilic addition, with the halogen acting as electrophile. In that regard, it resembles the halogen addition reaction and proceeds with anti addition, leaving the newly added X and OH groups in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. Among the simplest carbocations are the methenium (a carbenium ion), methanium (a carbonium ion), acylium ions , and Vinyl cation, vinyl cations. Until the early 1970s, carbocations were called ''carbonium ions''. This nomenclature was proposed by George Andrew Olah, G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a Three-center two-electron bond, three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diastereoselectivity
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is, excluding the opposing enantiomer). Diastereome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Single Bond
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond. When shared, each of the two electrons involved is no longer in the sole possession of the Atomic orbital, orbital in which it originated. Rather, both of the two electrons spend time in either of the orbitals which overlap in the bonding process. As a Lewis structure, a single bond is denoted as AːA or A-A, for which A represents an element. In the first rendition, each dot represents a shared electron, and in the second rendition, the bar represents both of the electrons shared in the single bond. A covalent bond can also be a double bond or a triple bond. A single bond is weaker than either a double bond or a triple bond. This difference in strength can be explained by examining the component bonds of which each of these types of covalent bonds consists (Moo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in the structure. For example, , , and are isoelectronic, while and = are not. This definition is sometimes termed valence isoelectronicity. Definitions can sometimes be not as strict, sometimes requiring identity of the total electron count and with it the entire electronic configuration. More usually, definitions are broader, and may extend to allowing different numbers of atoms in the species being compared.A. A. Aradi & T. P. Fehlner, "Isoelectronic Organometallic Molecules", in F. G. A. Stone & Robert West (eds.) ''Advances in Organometallic Chemistry Vol. 30'' (1990), Chapter 5 (at p. 190google books link/ref> The importance of the concept lies in identifying significantly related species, as pairs or series. Isoelectronic specie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |