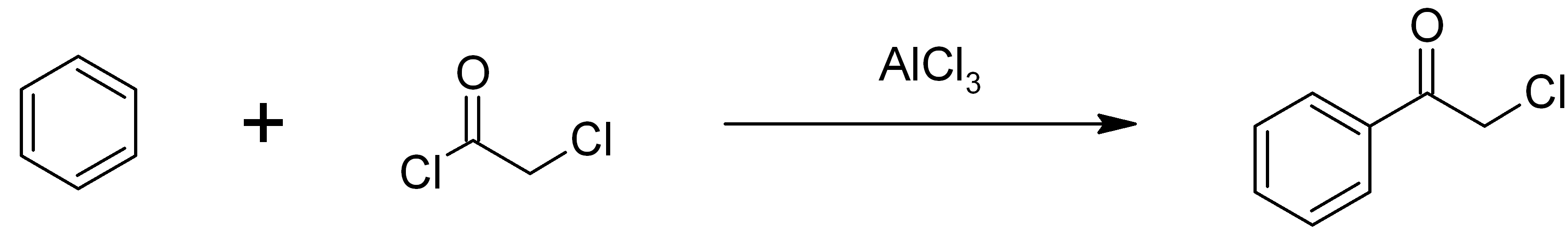|
Bromomethyl Ethyl Ketone
Bromomethyl ethyl ketone is a brominated ketone with lachrymatory effects. It was used as a chemical warfare agent in World War I. Bromomethyl ethyl ketone was developed as an alternative to bromoacetone, because acetone, the precursor to bromoacetone, was required for explosives production. See also *Bromoacetone *Chloroacetone *Iodoacetone *Bromobenzyl cyanide *Chloroacetophenone *Ethyl bromoacetate *Ethyl iodoacetate Ethyl iodoacetate is an organic compound with the chemical formula . It is a derivative of ethyl acetate. Under normal conditions, the compound is a clear, light yellow to orange liquid. Applications Used by the British during World War I, it was ... References Lachrymatory agents Ketones Organobromides World War I chemical weapons {{Organohalide-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoacetone
Bromoacetone is an organic compound with the formula . It is a colorless liquid although impure samples appear yellow or even brown. It is a lachrymatory agent and a precursor to other organic compounds. Occurrence in nature Bromoacetone is present (less than 1%) in the essential oil of a seaweed (''Asparagopsis taxiformis'') from the vicinity of the Hawaiian Islands. Synthesis Bromoacetone is available commercially, sometimes stabilized with magnesium oxide. It was first described in the 19th century, attributed to N. Sokolowsky. Bromoacetone is prepared by combining bromine and acetone, with catalytic acid. As with all ketones, acetone enolizes in the presence of acids or bases. The alpha carbon then undergoes electrophilic substitution with bromine. The main difficulty with this method is over-bromination, resulting in di- and tribrominated products. If a base is present, bromoform is obtained instead, by the haloform reaction. Applications It was used in World War I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a characteristic pungent odor. Acetone is miscibility, miscible with properties of water, water and serves as an important organic solvent in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and for production of methyl methacrylate and bisphenol A, which are precursors to widely used plastics.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in organic chemistry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoacetone
Bromoacetone is an organic compound with the formula . It is a colorless liquid although impure samples appear yellow or even brown. It is a lachrymatory agent and a precursor to other organic compounds. Occurrence in nature Bromoacetone is present (less than 1%) in the essential oil of a seaweed (''Asparagopsis taxiformis'') from the vicinity of the Hawaiian Islands. Synthesis Bromoacetone is available commercially, sometimes stabilized with magnesium oxide. It was first described in the 19th century, attributed to N. Sokolowsky. Bromoacetone is prepared by combining bromine and acetone, with catalytic acid. As with all ketones, acetone enolizes in the presence of acids or bases. The alpha carbon then undergoes electrophilic substitution with bromine. The main difficulty with this method is over-bromination, resulting in di- and tribrominated products. If a base is present, bromoform is obtained instead, by the haloform reaction. Applications It was used in World War I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroacetone
Chloroacetone is a chemical compound with the chemical formula, formula . At Standard temperature and pressure, STP it is a colorless liquid with a pungent odor. On exposure to light, it turns to a dark yellow-amber color. It was used as a lachrymatory agent, tear gas in World War I. Synthesis Chloroacetone may be synthesized from the reaction between chlorine and diketene, or by the chlorination of acetone. Applications Chloroacetone is used to make dye couplers for colour photography, and is an intermediate in chemical manufacturing. It is also used in the Feist-Benary synthesis of furans. : *Reaction of phenoxide with chloroacetone gives phenoxyacetone, which is used to make a wide variety of different pharmaceuticals. A catalytic amount of potassium iodide is also necessary to facilitate a Finkelstein reaction. Purification Chloroacetone purchased from commercial suppliers contains 5% impurities including mesityl oxide, which is not removed by distillation. Mesityl oxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodoacetone
Iodoacetone is an organoiodine compound with the chemical formula The substance is a colorless liquid under normal conditions, soluble in ethanol. Synthesis The reaction of acetone Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ... and iodine produces iodoacetone. The reaction is typically acid catalysed and first order with respect to acetone and the acid catalyst: : See also * Bromoacetone * Chloroacetone * Fluoroacetone * Thioacetone References {{Chemical agents Organoiodides Lachrymatory agents Ketones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromobenzyl Cyanide
Bromobenzyl cyanide (BBC), also known in the military idiom as camite (CA), is an obsolete lachrymatory agent introduced in World War I by the Allied Powers, being a standard agent, along with chloroacetophenone, adopted by the CWS. When implemented in World War I, it revolutionized the use of tear agents due to their extreme potency. BBC is toxic like chlorine gas. An application for bromobenzyl cyanide is in Hoch's synthesis of diphenylacetonitrile. See also * Chloroacetophenone *CR gas *CS gas *Lachrymatory agent Tear gas, also known as a lachrymatory agent or lachrymator (), sometimes colloquially known as "mace" after the early commercial self-defense spray, is a chemical weapon that stimulates the nerves of the lacrimal gland in the eye to produce ... References External links * * {{aromatic-stub Benzene derivatives Lachrymatory agents Nitriles Organobromides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroacetophenone
Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN. It should not be confused with cyanide, another agent used in chemical warfare, which has the chemical structure CN−. Chloroacetophenone is thermally stable, and is the only tear agent that is distillable at ambient conditions. Preparation Chloroacetophenone was first synthetized by Carl Graebe in 1871 by passing chlorine into boiling acetophenone. Phenacyl chloride is readily available and was first prepared by chlorination of acetophenone vapour. It may also be synthesized by the Friedel-Crafts acylation of benzene using chloroacetyl chloride, with an aluminium chloride catalyst: : Riot control agent It was investigated, but not used, during the First and Second World Wars (it was used as a "green agent" by the former Japanese milit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Bromoacetate
Ethyl bromoacetate is the chemical compound with the formula . It is the ethyl ester of bromoacetic acid and is prepared in two steps from acetic acid. It is a lachrymator and has a fruity, pungent odor. It is also a highly toxic alkylating agent and may be fatal if inhaled. Applications Ethyl bromoacetate is listed by the World Health Organization as a riot control agent, and was first employed for that purpose by French police in 1912. The French army used rifle grenades 'grenades lacrymogènes' filled with this gas against the Germans beginning in August 1914, but the weapons were largely ineffective, even though ethyl bromoacetate is twice as toxic as chlorine. In the early months of the war the British also used the weaponized use of tear gas agents and more toxic gasses including sulfur dioxide. The German army then used these attacks to justify their subsequent employment of it as odorant or warning agent in odorless, toxic gases and chemical weapons in 1915 under the Ger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Iodoacetate
Ethyl iodoacetate is an organic compound with the chemical formula . It is a derivative of ethyl acetate. Under normal conditions, the compound is a clear, light yellow to orange liquid. Applications Used by the British during World War I, it was codenamed SK gas, for the initials of South Kensington, where it was developed. Like many alkyl iodides, ethyl iodoacetate is an alkylating agent, which makes it useful in organic synthesis, yet toxic. Ethyl iodoacetate is also a lachrymatory agent Tear gas, also known as a lachrymatory agent or lachrymator (), sometimes colloquially known as "mace" after the early commercial self-defense spray, is a chemical weapon that stimulates the nerves of the lacrimal gland in the eye to produce .... References {{Chemical warfare Lachrymatory agents Organoiodides Ethyl esters Iodoacetates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lachrymatory Agents , a small vessel of terracotta or glass found in Roman and late Greek tombs, thought to have been used to collect the tears of mourners at funerals
{{disambiguation ...
Lachrymatory or lacrymatory may refer to: * Something that has the effect of ''lachrymation'', causing the secretion of tears * Tear gas, known formally as a ''lachrymatory agent'' or ''lachrymator'' * A lacrymatory A lacrymatory, lachrymatory or lacrimarium (from the Latin ''lacrima'', ' tear') is a small vessel of terracotta or, more frequently, of glass, found in Roman and late Greek tombs, and formerly supposed to have been bottles into which mourners ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' are methyl), with the formula . Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, ''e.g.'', testosterone, and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered retained IUPAC names, although some introd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organobromides
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon Chemical bond, bonded to bromine. The most pervasive is the naturally produced bromomethane. One prominent application of synthetic organobromine compounds is the use of polybrominated diphenyl ethers as fire-retardants, and in fact fire-retardant manufacture is currently the major industrial use of the element bromine. A variety of minor organobromine compounds are found in nature, but none are biosynthesized or required by mammals. Organobromine compounds have fallen under increased scrutiny for their environmental impact. General properties Most organobromine compounds, like most organohalogens, organohalide compounds, are relatively nonpolar. Bromine is more electronegative than carbon (2.9 vs 2.5). Consequently, the carbon in a carbon–bromine bond is electrophilic, i.e. alkyl bromides are alkylating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |