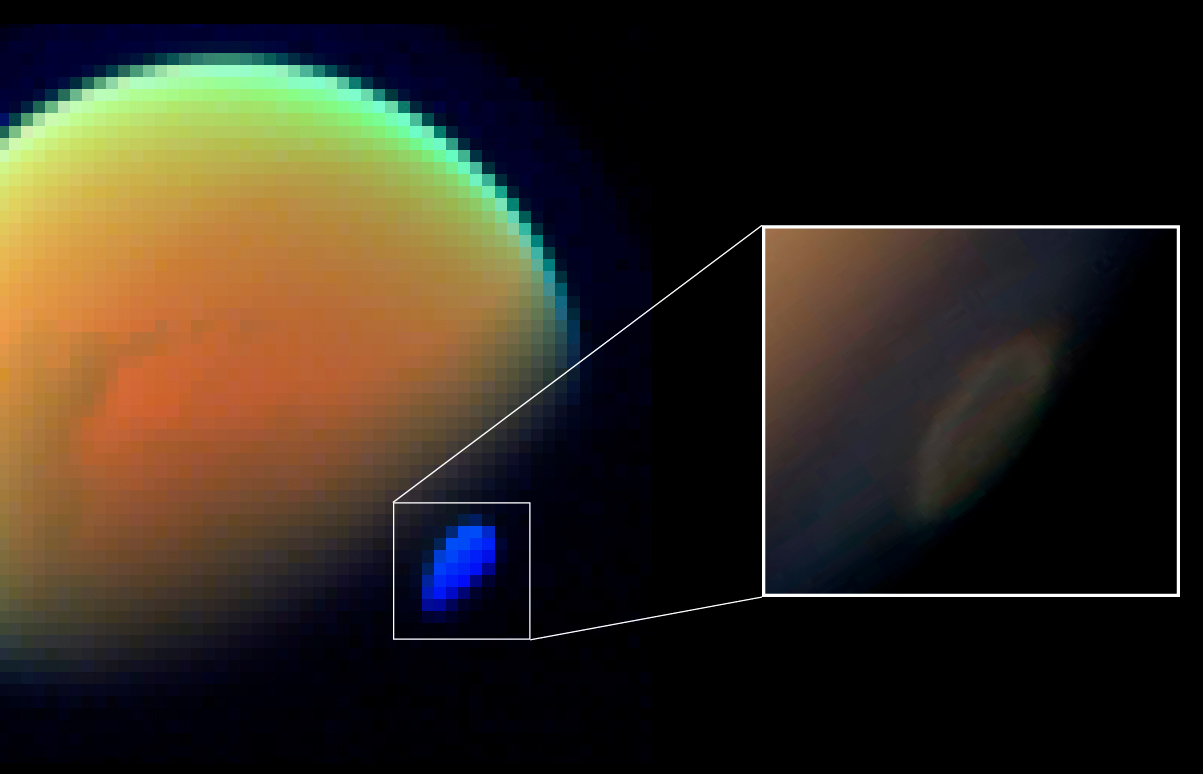
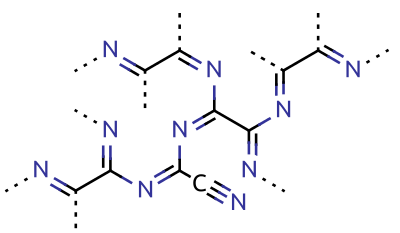
|
Bromine Cyanide
Cyanogen bromide is the inorganic compound with the formula BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides (cuts the C-terminus of methionine), and synthesize other compounds. The compound is classified as a pseudohalogen. Synthesis, basic properties, and structure The carbon atom in cyanogen bromide is bonded to bromine by a single bond and to nitrogen by a triple bond (i.e. ). The compound is linear and polar, but it does not spontaneously ionize in water. It dissolves in both water and polar organic solvents. Cyanogen bromide can be prepared by oxidation of sodium cyanide with bromine, which proceeds in two steps via the intermediate cyanogen (): : : When refrigerated the material has an extended shelflife. Like some other cyanogen compounds, cyanogen bromide undergoes an exothermic trimerisation to cyanuric bromide (). This reaction is catalyzed by traces of bromine, metal salts, acids and bases. For this reason, e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued Precursor (chemistry), precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its Volatility (chemistry), volatile nature. A solution of hydrogen cyanide in water (molecule), water, represented as HCN(aqueous, aq), is called ''hydrocyanic acid''. The Salt (chemistry), salts of the cyanide anion are known as cyanides. Whether hydrogen cyanide is an organic compound or not is a topic of debate among chemists, and opinions vary from author to author. Traditionally, it is considered ino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CNBr Activated Matrices Reaction
Cyanogen bromide is the inorganic compound with the formula BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides (cuts the C-terminus of methionine), and synthesize other compounds. The compound is classified as a pseudohalogen. Synthesis, basic properties, and structure The carbon atom in cyanogen bromide is bonded to bromine by a single bond and to nitrogen by a triple bond (i.e. ). The compound is linear and polar, but it does not spontaneously ionize in water. It dissolves in both water and polar organic solvents. Cyanogen bromide can be prepared by oxidation of sodium cyanide with bromine, which proceeds in two steps via the intermediate cyanogen (): : : When refrigerated the material has an extended shelflife. Like some other cyanogen compounds, cyanogen bromide undergoes an exothermic trimerisation to cyanuric bromide (). This reaction is catalyzed by traces of bromine, metal salts, acids and bases. For this reason, e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanamide
Cyanamide is an organic compound with the formula C N2 H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a nitrile group attached to an amino group. Derivatives of this compound are also referred to as cyanamides, the most common being calcium cyanamide (CaCN2). Tautomers and self-condensations Containing both a nucleophilic and electrophilic site within the same molecule, cyanamide undergoes various reactions with itself. Cyanamide exists as two tautomers, one with the connectivity N≡C–NH2 and the other with the formula HN=C=NH (" carbodiimide" tautomer). The N≡C–NH2 form dominates, but in a few reactions (e.g. silylation) the diimide form appears to be important. Cyanamide dimerizes to give 2-cyanoguanidine (dicyandiamide). This dimerization is hindered or reversed by acids and is inhibited by low temperatures. The cyclic trime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide Bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein chain. It can also be called a eupeptide bond to distinguish it from an isopeptide bond, which is another type of amide bond between two amino acids. Synthesis When two amino acids form a '' dipeptide'' through a ''peptide bond'', it is a type of condensation reaction. In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. One loses a hydrogen and oxygen from its carboxyl group (COOH) and the other loses a hydrogen from its amino group (NH2). This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (−CO−NH−). The two joined amino acids are called a dipeptide. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrobromic Acid
Hydrobromic acid is an aqueous solution of hydrogen bromide. It is a strong acid formed by dissolving the diatomic molecule hydrogen bromide (HBr) in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at and contains 47.6% HBr by mass, which is 8.77 mol/L. Hydrobromic acid is one of the strongest mineral acids known. Uses Hydrobromic acid is mainly used for the production of inorganic bromides, especially the bromides of zinc, calcium, and sodium. It is a useful reagent for generating organobromine compounds. Certain ethers are cleaved with HBr. It also catalyzes alkylation reactions and the extraction of certain ores. Industrially significant organic compounds prepared from hydrobromic acid include allyl bromide, tetrabromobis(phenol), and bromoacetic acid. HBr participates in anti-Markovnikov hydrohalogenation of alkenes in the presence of peroxides. The resulting 1-bromoalkanes are versatile alkylating agents, giving rise to fatty amines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanic Acid
Isocyanic acid is a chemical compound with the structural formula HNCO, which is often written as . It is a colourless, volatile and poisonous gas, condensing at 23.5 °C. It is the predominant tautomer and an isomer of cyanic acid ''(aka. cyanol)'' (), and the monomer of cyanuric acid. The derived anion of isocyanic acid is the same as the derived anion of cyanic acid, and that anion is , which is called cyanate. The related functional group is isocyanate; it is distinct from cyanate (), fulminate (), and nitrile oxide (). Isocyanic acid was discovered in 1830 by Justus von Liebig and Friedrich Wöhler. Isocyanic acid is the simplest stable chemical compound that contains carbon, hydrogen, nitrogen, and oxygen, the four most commonly found elements in organic chemistry and biology. It is the only fairly stable one of the four linear isomers with molecular formula HOCN that have been synthesized, the others being cyanic acid (cyanol, ) and the elusive fulminic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolyzed
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanuric Bromide
Cyanuric bromide is a heterocyclic compound with formula C3N3Br3. It contains a six-membered ring of alternating nitrogen and carbon atoms, with a bromine atom attached to each carbon. It is formed by the spontaneous trimerisation of cyanogen bromide. : Reactions Cyanuric bromide can be used to synthesize substituted triazines. For example it reacts with anilines to form derivatives of melamine. With ammonia, melamine is produced. Primary or secondary amines react. Cyanuric trihydrazide is produced in the reaction with hydrazine. When heated with urea at 140 °C, ammelide is formed. Cyanuric bromide reacts with water, particularly in alkaline conditions to cyanuric acid and hydrogen bromide. Cyanuric bromide can add bromine to other compounds and when it is heated with acetic acid, acetyl bromide is produced. Formation Cyanuric bromide can form in a reaction with potassium ferrocyanide with bromine at 200 °C. The trimerization reaction of cyanogen bromide (BrCN) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanogen
Cyanogen is the chemical compound with the chemical formula, formula . Its structure is . The simplest stable carbon nitride, it is a Transparency and translucency, colorless and highly toxic gas with a pungency, pungent odor. The molecule is a pseudohalogen. Cyanogen molecules are linear molecular geometry, linear, and consist of two CN groups ‒ analogous to diatomic halogen molecules, such as chlorine, Cl, but far less oxidizing. The two cyanide, cyano groups are bonded together at their carbon atoms, though other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide () (but see also ''Cyano radical''). When burned at increased pressure with oxygen, it is possible to get a blue tinted flame, the temperature of which is about 4800°C (a higher temperature is possible with ozone). It is as such regarded as the gas with the second highest temperature of burning (after dicyanoacetylene). Cyanogen is the anhy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Cyanide
Sodium cyanide is a compound with the formula Na C N and the structure . It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. It is a moderately strong base. Production and chemical properties Sodium cyanide is produced by treating hydrogen cyanide with sodium hydroxide: : Worldwide production was estimated at 500,000 tons in the year 2006. Formerly it was prepared by the Castner process involving the reaction of sodium amide with carbon at elevated temperatures. : The structure of solid NaCN is related to that of sodium chloride. The anions and cations are each six-coordinate. Potassium cyanide (KCN) adopts a similar structure. When treated with acid, it forms the toxic gas hydrogen cyanide: : Because the salt is derived from a weak acid, sodium cyanide readily reverts to HCN by hydrolysis; the moist solid emits smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |