|
Bredt's Rule
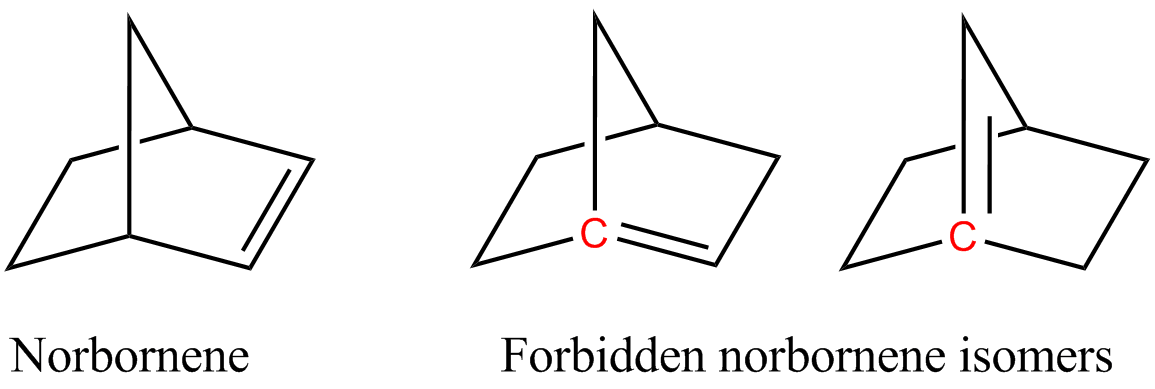
In organic chemistry, an anti-Bredt molecule is a Bridged compound, bridged molecule with a double bond at the Bicyclic molecule, bridgehead. Bredt's rule is the empirical observation that such molecules only form in large ring systems. For example, two of the following norbornene isomers violate Bredt's rule, and are too unstable to prepare: The rule is named after Julius Bredt, who first discussed it in 1902 and codified it in 1924. There are a few instances where the anti-Bredt phenomenon is mentioned, but the isolation of these molecules is difficult, so they are typically trapped in situ. In pioneering studies, Wiseman, Keese, Wiberg, and others validated the intermediacy of anti-Bredt olefins beginning in the 1960s. Authors such as Mehta (2002) and Khan (2015) also obtained some possible support for the intermediacy of anti-Bredt olefins. In 2024, Neil Garg and his team demonstrated that the formation of anti-Bredt molecules is possible, even if only as short-lived intermed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen. Organic chemistry In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure. Typical heteroatoms are nitrogen (N), oxygen (O), sulfur (S), phosphorus (P), chlorine (Cl), bromine (Br), and iodine (I), as well as the metals lithium (Li) and magnesium (Mg). Proteins It can also be used with highly specific meanings in specialised contexts. In the description of protein structure, in particular in the Protein Data Bank file format, a heteroatom record (HETATM) describes an atom as belonging to a small molecule cofactor rather than being part of a biopolymer chain. Zeolites In the context of zeolites, the term ''heteroatom'' refers to partial isomorphous substitution of the typical framework atoms (silicon, aluminium, and phosphorus) by other elements such as beryllium, vanadium, and chromium. The goal i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature (journal)
''Nature'' is a British weekly scientific journal founded and based in London, England. As a multidisciplinary publication, ''Nature'' features Peer review, peer-reviewed research from a variety of academic disciplines, mainly in science and technology. It has core editorial offices across the United States, continental Europe, and Asia under the international scientific publishing company Springer Nature. ''Nature'' was one of the world's most cited scientific journals by the Science Edition of the 2022 ''Journal Citation Reports'' (with an ascribed impact factor of 50.5), making it one of the world's most-read and most prestigious academic journals. , it claimed an online readership of about three million unique readers per month. Founded in the autumn of 1869, ''Nature'' was first circulated by Norman Lockyer and Alexander MacMillan (publisher), Alexander MacMillan as a public forum for scientific innovations. The mid-20th century facilitated an editorial expansion for the j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iminoether
Carboximidates (or more general imidates) are organic compounds, which can be thought of as esters formed between an imidic acid () and an alcohol, with the general formula . They are also known as imino ethers, since they resemble imines () with an oxygen atom connected to the carbon atom of the C=N double bond. Synthesis Imidates may be generated by a number of synthetic routes, but are in general formed by the Pinner reaction. This proceeds via the acid catalyzed attack of nitriles by alcohols. Imidates produced in this manner are formed as their hydrochloride salts, which are sometimes referred to as Pinner salts. Carboximidates are also formed as intermediates in the Mumm rearrangement and the Overman rearrangement. Imidate/amidate anions An amidate/imidate anion is formed upon deprotonation of an amide or imidic acid. Since amides and imidic acids are tautomers, they form the same anion upon deprotonation. The two names are thus synonyms describing the same anion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
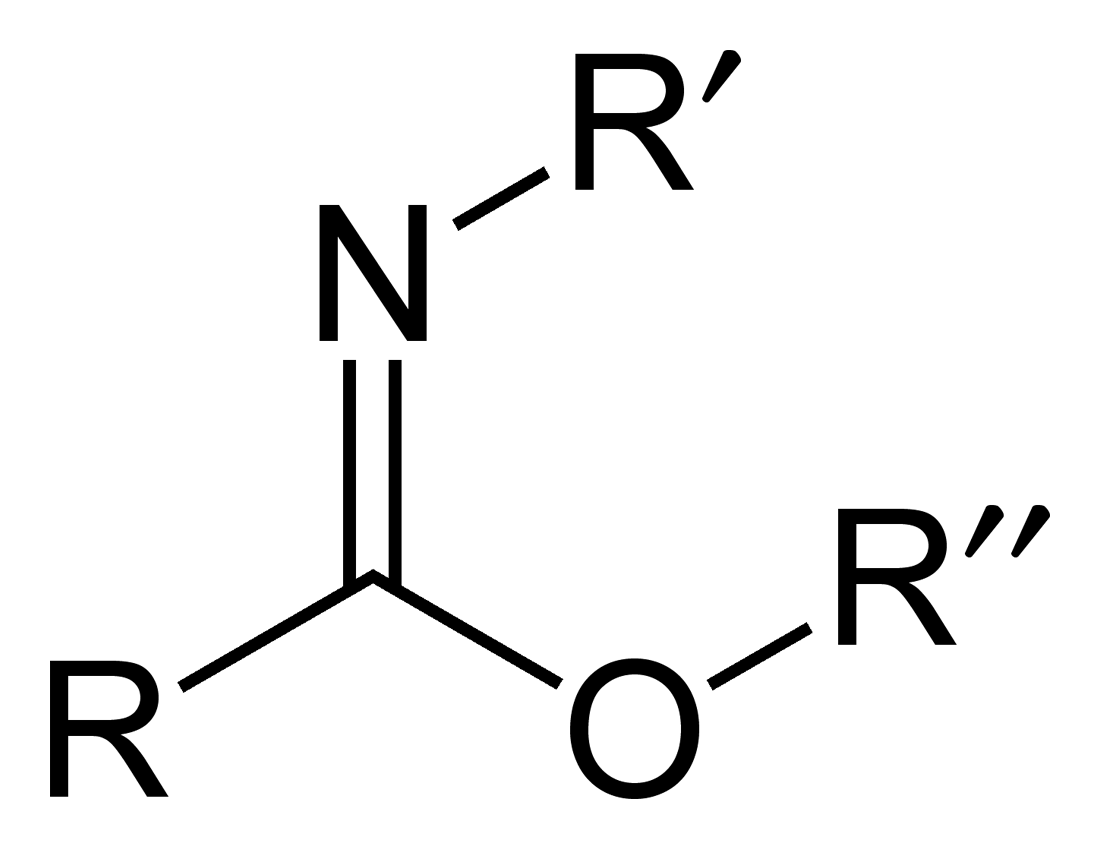
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the Polymer backbone, main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a Derivative (chemistry), derivative of a carboxylic acid () with the hydroxyl group () replaced by an amino group (); or, equivalently, an acyl group, acyl (alkanoyl) group () joined to an amino group. Common amides are formamide (), acetamide (), benzamide (), and dimethylformamide (). Some uncommon examples of amides are ''N''-chloroacetamide () and chloroformamide (). Amides are qualified as primary (chemistry), primary, secondary (chemistry), secondary, and tertiary (chemistry), tertiary according to the number of acyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-quinuclidonium Tetrafluoroborate
Quinuclidones are a class of bicyclic organic compounds with chemical formula C7H11NO with two structural isomers for the base skeleton 3-quinuclidone and 2-quinuclidone. 3-Quinuclidone (1-azabicyclo .2.2ctan-3-one) is an uneventful molecule that can be synthesized as the hydrochloric acid salt by a Dieckman condensation: The other isomer, 2-quinuclidone, appears equally uneventful, but in fact it has defied synthesis until 2006. The reason is that this molecule is very unstable because its amide group has the amine lone pair and the carbonyl group not properly aligned, as may be expected for an amide, as a result of steric strain. This behaviour is predicted by Bredt's Rule, and formal amide group resembles in fact an amine, as evidenced by the ease of salt formation. The organic synthesis of the tetrafluoroborate salt of 2-quinuclidone is a six-step affair starting from norcamphor the final step being an azide - ketone Schmidt reaction (38% yield): This compound rapid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance (chemistry)
In chemistry, resonance, also called mesomerism, is a way of describing Chemical bond, bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. The resonance hybrid is the accurate structure for a molecule or ion; it is an average of the theoretical (or hypothetical) contributing structures. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is suffi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene"/"alkene" and the "-ol". Many kinds of enols are known. Keto–enol tautomerism refers to a chemical equilibrium between a "keto" form (a carbonyl, named for the common ketone case) and an enol. The interconversion of the two forms involves the transfer of an alpha hydrogen atom and the reorganisation of bonding electrons. The keto and enol forms are tautomers of each other. Enolization Organic esters, ketones, and aldehydes with an α-hydrogen ( bond adjacent to the carbonyl group) often form enols. The reaction involves migration of a proton () from carbon to oxygen: : In the case of ketones, the conversion is called a keto-enol tautomerism, although this name is often more generally applied to all such tautomerizations. Usua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Tetranorbornyl
In organometallic chemistry, metal tetranorbornyls are compounds with the formula M(nor)4 (M = a metal in a +4 oxidation state) (1-nor = 4bicyclo[2.2.1]hept-1-yl) and are one of the largest series of tetraalkyl complexes derived from identical ligands. Metal tetranorbornyls display uniform stoichiometry, Low spin, low-spin configurations, and high Stability constants of complexes, stability, which can be attributed to their +4 oxidation state metal center. The stability of metal tetranorbornyls is predominately considered to be derived from the unfavorable Beta-Hydride elimination, β-hydride elimination. Computational calculations have determined that London dispersion force, London dispersion effects significantly contribute to the stability of metal tetranorbornyls. Specifically, Fe(nor)4 has a stabilization of 45.9 kcal/mol−1. Notable metal tetranorbornyls are those synthesized with metal centers of cobalt, manganese, or iron. Preparation Traditionally, metal tetranorbor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Elimination
Beta (, ; uppercase , lowercase , or cursive ; or ) is the second letter of the Greek alphabet. In the system of Greek numerals, it has a value of 2. In Ancient Greek, beta represented the voiced bilabial plosive . In Modern Greek, it represents the voiced bilabial fricative while in borrowed words is instead commonly transcribed as μπ. Letters that arose from beta include the Roman letter and the Cyrillic letters and . Name Like the names of most other Greek letters, the name of beta was adopted from the acrophonic name of the corresponding letter in Phoenician, which was the common Semitic word ('house', compare and ). In Greek, the name was , pronounced in Ancient Greek. It is spelled in modern monotonic orthography and pronounced . History The letter beta was derived from the Phoenician letter beth . The letter Β had the largest number of highly divergent local forms. Besides the standard form (either rounded or pointed, ), there were forms as varied as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Alkyl Complexes
Transition metal alkyl complexes are coordination complexes that contain a bond between a transition metal and an alkyl ligand. Such complexes are not only pervasive but are of practical and theoretical interest. Scope Most metal alkyl complexes contain other, non-alkyl ligands. Great interest, mainly theoretical, has focused on the homoleptic complexes. Indeed, the first reported example of a complex containing a metal-sp3 carbon bond was the homoleptic complex diethylzinc. Other examples include hexamethyltungsten, tetramethyltitanium, and tetranorbornylcobalt. : 200 px, Structure of diethylzinc. The Zn-C bonds measure 194.8(5) pm, while the C-Zn-C angle is slightly bent with 176.2(4)°. Mixed ligand, or heteroleptic, complexes containing alkyls are numerous. In nature, vitamin B12 and its many derivatives contain reactive Co-alkyl bonds. : 180 px, Hexamethyltungsten is an example of a "homoleptic" (all ligands being the same) metal alkyl complex. Preparation Metal al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elimination Reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1cB-elimination reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism, Ei mechanism. E2 mechanism The E2 mechanism, where E2 stands for bimolecular elimination, involves a one-step mechanism in which ''carbon-hydrogen'' and ''carbon-halogen'' bonds break to form a double bond (''C=C molecular geometry, Pi bond''). The specifics of the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |