|
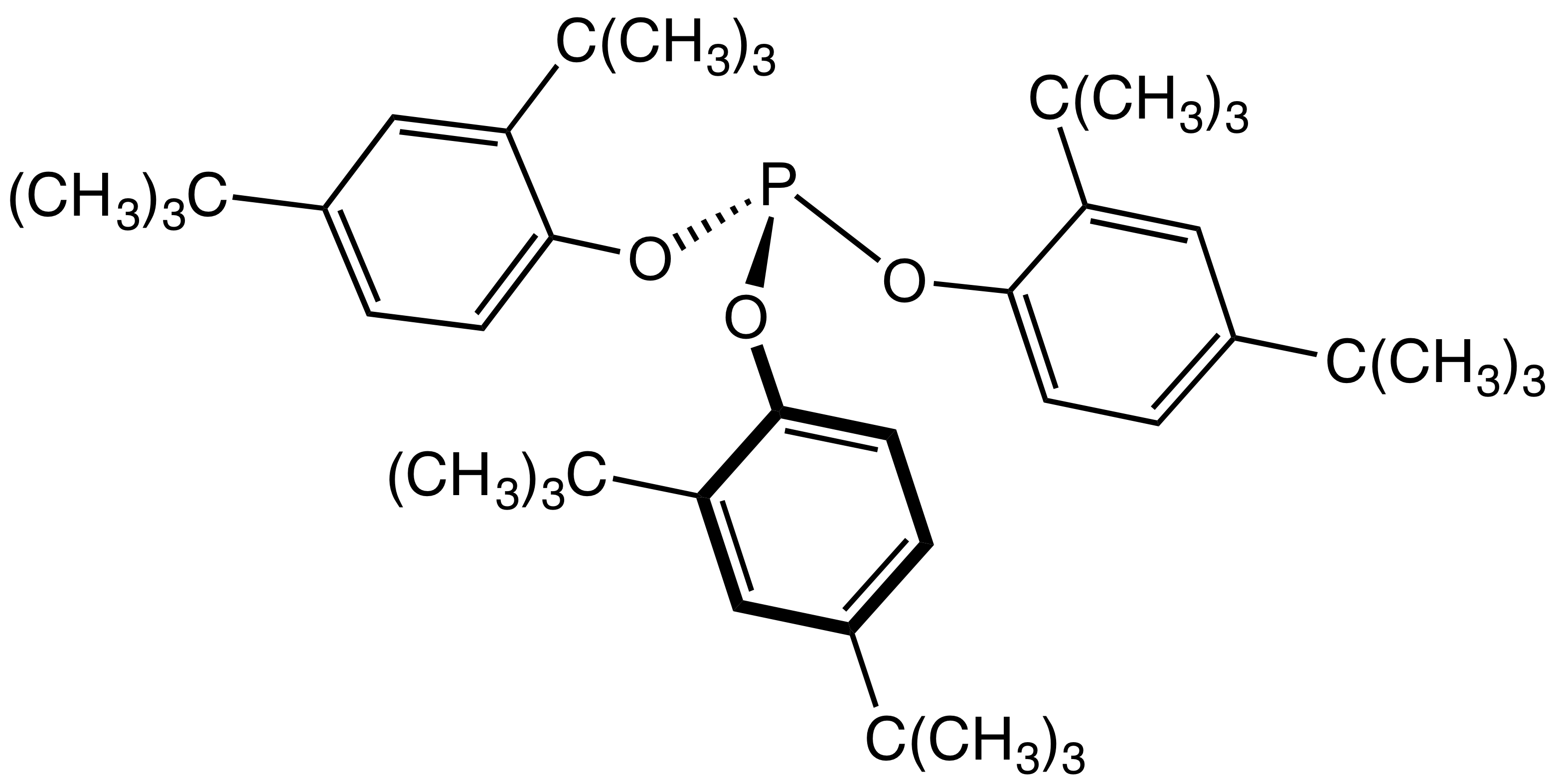
BiPhePhos
BiPhePhos is an organophosphorus compound that is used as a ligand in homogeneous catalysis. Classified as a diphosphite, BiPhePhos is derived from three 2,2'-biphenol groups, which constrain its shape in such a way to confer high selectivity to derived catalysts. Originally described by workers at Union Carbide, it has become a standard ligand in hydroformylation.{{cite journal, doi=10.1021/OM950549K, title=Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization, journal=Organometallics, volume=15, issue=2, pages=835–847, year=1996, last1=Van Rooy, first1=Annemiek, last2=Kamer, first2=Paul C. J., last3=Van Leeuwen, first3=Piet W. N. M., last4=Goubitz, first4=Kees, last5=Fraanje, first5=Jan, last6=Veldman, first6=Nora, last7=Spek, first7=Anthony L., url=http://dare.uva.nl/personal/pure/en/publications/bulky-diphosphite-modified-rhodium-catalyst-hydroformylation-and-characterization(c06c2654-cecb-4e97-84ba-f1fdfb51ad35).html See also *2,2'-Biphenylene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphite
The general structure of a phosphite ester showing the lone pairs on the P In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids. Synthesis ;From PCl3 Phosphite esters are typically prepared by treating phosphorus trichloride with an alcohol. Depending on the synthetic details, this alcoholysis can give the diorganophosphites: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Alternatively, when the alcoholysis is conducted in the presence of proton acceptors, one obtains the C3-symmetric trialkoxy derivat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,2'-Biphenylene Phosphorochloridite
2,2′-Biphenylene phosphorochloridite is the name for a polycyclic organophosphorus compound with the formula C12H8O2PCl. It is a precursor to di phosphite ligands such as BiPhePhos by reaction with suitable diols. 2,2′-Biphenylene phosphorochloridites, which is a white solid, is prepared from 2,2′- biphenol and phosphorus trichloride. It is prepared by the reaction of 2,2′-biphenol and phosphorus trichloride Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic a ....{{cite journal, doi=10.1021/OM950549K, title=Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization, journal=Organometallics, volume=15, pages=835–847, year=1996, last1=Van Rooy, first1=Annemiek, last2=Kamer, first2=Paul C. J., last3=Van Leeuwen, first3=Piet W. N. M., last4=Goubitz, first ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogeneous Catalysis
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid-gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts. Examples Acid catalysis The proton is a pervasive homogeneous catalyst because water is the most common solvent. Water forms protons by the process of self-ionization of water. In an illustrative case, acids accelerate (catalyze) the hydrolysis of esters: :CH3CO2CH3 + H2O CH3CO2H + CH3OH At neutral pH, aqueous solutions of m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biphenol
In organic chemistry, a biphenol refers to compounds with the formula (C6H4OH)2. Such compounds formally result from the coupling of two phenols. {{short description, Chemical compound Three symmetrical isomers of biphenol exist: *2,2'-Biphenol ( RN 1806-29-7) m.p. 109 °C * 3,3'-Biphenol (RN 612-76-0) m.p. 124.8 °C *4,4'-Biphenol 4,4′-Biphenol is an organic compound which is a phenolic derivative of biphenyl. It is a colorless solid. 4,4′-Biphenol is prepared by dealkylation of the tetra-''t''-butyl derivative, generated by the oxidative coupling of 2,6-di-''tert''-b ... (RN 92-88-6) m.p. 283 °C Additionally, three unsymmetrical isomers of biphenol exist: * 2,3'-Biphenol (RN 31835-45-7) * 2,4'-Biphenol (RN 611-62-1) m.p. 162-163 °C * 3,4'-Biphenol (RN 18855-13-5) m.p. 190 °C Phenols Biphenyls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Union Carbide
Union Carbide Corporation is an American chemical corporation wholly owned subsidiary (since February 6, 2001) by Dow Chemical Company. Union Carbide produces chemicals and polymers that undergo one or more further conversions by customers before reaching consumers. Some are high-volume commodities and others are specialty products meeting the needs of smaller markets. Markets served include paints and coatings, packaging, wire and cable, household products, personal care, pharmaceuticals, automotive, textiles, agriculture, and oil and gas. The company is a former component of the Dow Jones Industrial Average. Founded in 1917 as the Union Carbide and Carbon Corporation, from a merger with National Carbon Company, the company's researchers developed an economical way to make ethylene from natural gas liquids, such as ethane and propane, giving birth to the modern petrochemical industry. The company divested consumer products businesses Eveready and Energizer batteries, Glad bag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelating Agents
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast agents in MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |