|
Benodanil
Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates in both the citric acid cycle and oxidative phosphorylation. Histochemical analysis showing high succinate dehydrogenase in muscle demonstrates high mitochondrial content and high oxidative potential. In step 6 of the citric acid cycle, SQR catalyzes the oxidation of succinate to fumarate with the reduction of ubiquinone to ubiquinol. This occurs in the inner mitochondrial membrane by coupling the two reactions together. Structure Subunits Mitochondrial and many bacterial SQRs are composed of four structurally different subunits: two hydrophilic and two hydrophobic. The first two subunits, a flavoprotein (SDHA) and an iron-sulfur protein (SDHB), form a hydrophilic head where enzymatic activity of the complex takes place. SDHA co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helices
A helix (; ) is a shape like a cylindrical coil spring or the thread of a machine screw. It is a type of smoothness (mathematics), smooth space curve with tangent lines at a constant angle to a fixed axis. Helices are important in biology, as the DNA molecule is formed as double helix, two intertwined helices, and many proteins have helical substructures, known as alpha helix, alpha helices. The word ''helix'' comes from the Greek language, Greek word , "twisted, curved". A "filled-in" helix – for example, a "spiral" (helical) ramp – is a surface called a ''helicoid''. Properties and types The pitch of a helix is the height of one complete helix turn (angle), turn, measured parallel to the axis of the helix. A double helix consists of two (typically congruence (geometry), congruent) helices with the same axis, differing by a translation along the axis. A circular helix (i.e. one with constant radius) has constant band curvature and constant Torsion of curves, torsion. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
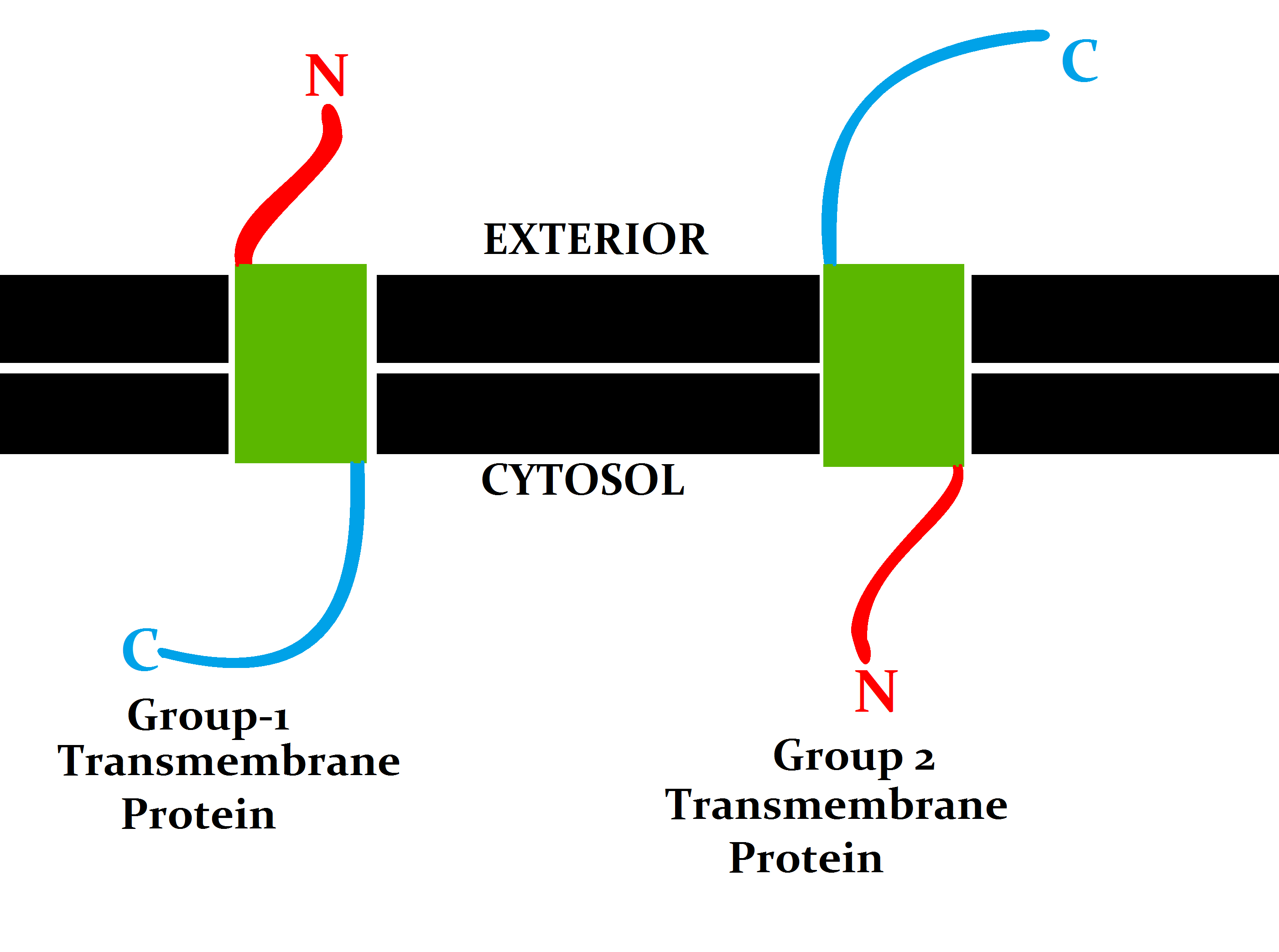
Transmembrane
A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them ( beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-pass membrane proteins, or as multipass membrane proteins. Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome B
Cytochrome b is a protein found in the membranes of aerobic cells. In eukaryotic mitochondria (inner membrane) and in aerobic prokaryotes, cytochrome b is a component of respiratory chain complex III () — also known as the bc1 complex or ubiquinol-cytochrome c reductase. In plant chloroplasts and cyanobacteria, there is a homologous protein, cytochrome b6, a component of the plastoquinone-plastocyanin reductase (), also known as the b6f complex. These complexes are involved in electron transport, the pumping of protons to create a proton-motive force ( PMF). This proton gradient is used for the generation of ATP. These complexes play a vital role in cells. Structure and function Cytochrome b/b6 is an integral membrane protein of approximately 400 amino acid residues that probably has 8 transmembrane segments. In plants and cyanobacteria, cytochrome b6 consists of two protein subunits encoded by the petB and petD genes. Cytochrome b/b6 non-covalently binds two heme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may include other proteins (resulting in a protein–protein interaction), enzyme substrates, second messengers, hormones, or allosteric modulators. The binding event is often, but not always, accompanied by a conformational change that alters the protein's function. Binding to protein binding sites is most often reversible (transient and non-covalent), but can also be covalent reversible or irreversible. Function Binding of a ligand to a binding site on protein often triggers a change in conformation in the protein and results in altered cellular function. Hence binding site on protein are critical parts of signal transduction pathways. Types of ligands include neurotransmitters, toxins, neuropeptides, and steroid hormones. Binding site ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be classified into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a " prosthetic group", which consists of a coenzyme that is tightly (or even covalently and, therefore, permanently) bound to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavin Adenine Dinucleotide
In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which may be in the form of FAD or flavin mononucleotide (FMN). Many flavoproteins are known: components of the succinate dehydrogenase complex, α-ketoglutarate dehydrogenase, and a component of the pyruvate dehydrogenase complex. FAD can exist in four redox states, which are the flavin-N(5)-oxide, quinone, semiquinone, and hydroquinone. FAD is converted between these states by accepting or donating electrons. FAD, in its fully oxidized form, or quinone form, accepts two electrons and two protons to become FADH2 (hydroquinone form). The semiquinone (FADH·) can be formed by either reduction of FAD or oxidation of FADH2 by accepting or donating one electron and one proton, respectively. Some proteins, however, generate and maintain a super ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalently
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term "covalence" was introduced by Irving Langmuir in 1919, with Nevil Sidgwick using "co-valent link" in the 1920s. Merriam-Webster dates the specific phrase ''covalent bond'' to 1939, recognizing its first known us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavoprotein
Flavoproteins are proteins that contain a nucleic acid derivative of riboflavin. These proteins are involved in a wide array of biological processes, including removal of radicals contributing to oxidative stress, photosynthesis, and DNA repair. The flavoproteins are some of the most-studied families of enzymes. Flavoproteins have either FMN ( flavin mononucleotide) or FAD ( flavin adenine dinucleotide) as a prosthetic group or as a cofactor. The flavin is generally tightly bound (as in adrenodoxin reductase, wherein the FAD is buried deeply). About 5-10% of flavoproteins have a covalently linked FAD. Based on the available structural data, FAD-binding sites can be divided into more than 200 different types. 90 flavoproteins are encoded in the human genome; about 84% require FAD and around 16% require FMN, whereas 5 proteins require both. Flavoproteins are mainly located in the mitochondria. Of all flavoproteins, 90% perform redox reactions and the other 10% are transferases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. The term ''hydrophobic''—which comes from the Ancient Greek (), "having a fear of water", constructed Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon. revised and augmented ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |