|
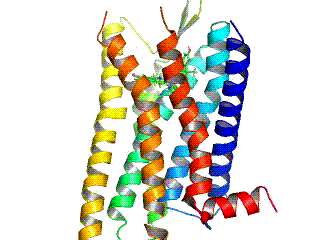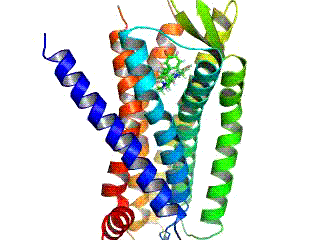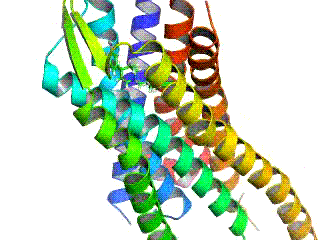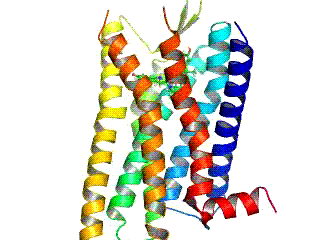
BMS‐986122
BMS‐986122 is a selective positive allosteric modulator (PAM) of the μ-opioid receptor (MOR). MOR PAMs like BMS-986122 could be useful as novel analgesics with reduced side effects compared to conventional opioid analgesics. However, the potential specifically of BMS-986121 and BMS-986122 as pharmaceutical drugs may be restricted due to their complex synthesis. Mechanism of action BMS-986122 can enhance the affinity and efficacy of various orthosteric MOR agonists, including the endogenous opioid peptides, for the MOR. However, its effects are dependent on the ligand, and in the case of morphine, it enhances efficacy without affecting affinity. BMS‐986122 has no MOR agonist activity, is selective for the MOR, and lacks PAM activity at the δ-opioid receptor (DOR). However, it has been identified as a silent allosteric modulator (SAM) of the DOR and κ-opioid receptor (KOR). Animal studies The drug has analgesic effects in animals. In contrast to MOR agonists, BMS-98 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
μ-opioid Receptor
The μ-opioid receptors (MOR) are a class of opioid receptors with a high affinity for enkephalins and beta-endorphin, but a low affinity for dynorphins. They are also referred to as μ(''mu'')-opioid peptide (MOP) receptors. The prototypical μ-opioid receptor agonist is morphine, the primary psychoactive alkaloid in opium and for which the receptor was named, with Mu (letter), mu being the first letter of Morpheus, the compound's namesake in the original Greek. It is an inhibitory G-protein coupled receptor that activates the Gi alpha subunit, Gi alpha subunit, inhibiting adenylate cyclase activity, lowering Cyclic adenosine monophosphate, cAMP levels. Structure The structure of the inactive μ-opioid receptor has been determined with the antagonists Beta-Funaltrexamine, β-FNA and alvimopan. Many structures of the active state are also available, with agonists including DAMGO, Β-Endorphin, β-endorphin, fentanyl and morphine. The structure with the agonist BU72 has the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ignavine
Ignavine is a naturally occurring diterpene alkaloid found in '' Aconiti tuber''. It has been reported to act as a μ-opioid receptor (MOR) positive allosteric modulator (PAM). The drug potentiated responses to the selective MOR agonist DAMGO at low concentrations but inhibited DAMGO at high concentrations. Ignavine alone has been found to produce analgesic effects in animals, but with a biphasic dose–response curve. Although described as a MOR PAM, other research suggests that ignavine is a ligand of the orthosteric site of the MOR and does not act as a PAM. Instead, it may be a MOR partial agonist. However, more research is necessary to clarify its MOR actions. Ignavine was first isolated by 1952 and its reported MOR PAM activity was first reported by 2016. See also * BMS‐986122 BMS‐986122 is a selective positive allosteric modulator (PAM) of the μ-opioid receptor (MOR). MOR PAMs like BMS-986122 could be useful as novel analgesics with reduced side effects compare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MS1 (drug)
MS1 is a positive allosteric modulator (PAM) of the μ-opioid receptor (MOR). It was developed from structural modification of the earlier MOR PAM BMS‐986122. The drug has been found to augment the affinity (pharmacology), affinity of the MOR agonist levomethadone ((''R'')-methadone) for the MOR by 7-fold ''in vitro'' and to potentiate activation of the MOR by levomethadone by 4-fold in a G protein assay. However, MS1 displays strong probe dependence, and while it potentiates the MOR agonists levomethadone and morphine, it had no effect on the affinity or potency (pharmacology), potency of the MOR agonists DAMGO or endomorphin-1. MS1 shows a preference for β-arrestin recruitment over G protein activation with endomorphin-1 exposure. The drug's actions are reportedly similar to those of BMS-986122, though its unclear if their mechanism of action, mechanisms of action are the same. MS1 shows potentiated analgesic effects with opioids in animals. It also did not worsen opioid wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BMS-986187
BMS-986187 is a positive allosteric modulator (PAM) of the δ-opioid receptor (DOR) and the κ-opioid receptor (KOR). The drug is highly potent as a DOR PAM, with an of 30nM. It has been found to increase the affinity of the endogenous peptide DOR agonist leu-enkephalin for the receptor by 32-fold. The drug has been found to act as a biased allosteric agonist of the DOR, activating G protein signaling ( = 301nM; Emax = 92%) but with little capacity to recruit β-arrestin ( = 579μM) (bias factor = 1787). Although a PAM, BMS-986187 is able to activate the DOR even in the absence of an orthosteric agonist, and as such, has been referred to as an "ago-PAM". Subsequent to its discovery, BMS-987187 was found to act as a potent KOR PAM as well. It is also a weak μ-opioid receptor (MOR) PAM ( = 3,000nM), but has 100-fold selectivity for potentiation of the DOR over the MOR. BMS-986187 has about 20- to 30-fold higher affinity for the conserved allosteric site on the DOR and KOR rel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Selectivity
In chemistry, binding selectivity is defined with respect to the binding of ligands to a substrate forming a complex. Binding selectivity describes how a ligand may bind more preferentially to one receptor than another. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate. Binding selectivity is of major importance in biochemistry and in chemical separation processes. Selectivity coefficient The concept of selectivity is used to quantify the extent to which one chemical substance, A, binds each of two other chemical substances, B and C. The simplest case is where the complexes formed have 1:1 stoichiometry. Then, the two interactions may be characterized by equilibrium constants and .The constant used here are ''association'' constants. ''Dissociation'' constants are used in some contexts. A dissociation constant is the reciprocal of an association constant. \begin \ce;& \quad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
κ-opioid Receptor
The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP for its ligand ketazocine, is a G protein-coupled receptor that in humans is encoded by the ''OPRK1'' gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction. The KOR is a type of opioid receptor that binds the opioid peptide dynorphin as the primary endogenous ligand (substrate naturally occurring in the body). In addition to dynorphin, a variety of natural alkaloids, terpenes and synthetic ligands bind to the receptor. The KOR may provide a natural addiction control mechanism, and therefore, drugs that target this receptor may have therapeutic potential in the treatment of addiction . There ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketamine
Ketamine is a cyclohexanone-derived general anesthetic and NMDA receptor antagonist with analgesic and hallucinogenic properties, used medically for anesthesia, depression, and pain management. Ketamine exists as its S- (esketamine) and R- (arketamine) two enantiomers and has antidepressant action likely involving additional mechanisms than NMDA antagonism. At anesthetic doses, ketamine induces a state of dissociative anesthesia, a trance-like state providing pain relief, sedation, and amnesia. Its distinguishing features as an anesthestic are preserved breathing and airway reflexes, stimulated heart function with increased blood pressure, and moderate bronchodilation. As an anesthetic, it is used especially in trauma, Emergency medical services, emergency, and Pediatrics, pediatric cases. At lower, sub-anesthetic doses, it is used as a treatment for pain and treatment-resistant depression. Ketamine is legally used in medicine but is also tightly controlled due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aconitum
''Aconitum'' (), also known as aconite, monkshood, wolfsbane, leopard's bane, devil's helmet, or blue rocket, is a genus of over 250 species of flowering plants belonging to the family (biology), family Ranunculaceae. These herbaceous perennial plants are chiefly native plant, native to the mountainous parts of the Northern Hemisphere in North America, Europe, and Asia, growing in the moisture-retentive but well-draining soils of mountain meadows. Most ''Aconitum'' species are extremely poisonous and must be handled very carefully. Several ''Aconitum'' Hybrid (biology), hybrids, such as the Arendsii form of ''Aconitum carmichaelii'', have won gardening awards—such as the Royal Horticultural Society's Award of Garden Merit. Some are used by florists. Etymology The name ''aconitum'' comes from the Greek word , which may derive from the Greek ''akon'' for dart (missile), dart or javelin, the tips of which were poisoned with the substance, or from ''akonae'', because of the ro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Opioid Receptor
Opioid receptors are a group of inhibitory G protein-coupled receptors with opioids as ligands. The endogenous opioids are dynorphins, enkephalins, endorphins, endomorphins and nociceptin. The opioid receptors are ~40% identical to somatostatin receptors (SSTRs). Opioid receptors are distributed widely in the brain, in the spinal cord, on peripheral neurons, and digestive tract. Discovery By the mid-1960s, it had become apparent from pharmacologic studies that opioids were likely to exert their actions at specific receptor sites, and that there were likely to be multiple such sites. Early studies had indicated that opiates appeared to accumulate in the brain. The receptors were first identified as specific molecules through the use of binding studies, in which opiates that had been labeled with radioisotopes were found to bind to brain membrane homogenates. The first such study was published in 1971, using 3H- levorphanol. In 1973, Candace Pert and Solomon H. Snyder publi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allosteric Site
In the fields of biochemistry and pharmacology an allosteric regulator (or allosteric modulator) is a substance that binds to a site on an enzyme or receptor distinct from the active site, resulting in a conformational change that alters the protein's activity, either enhancing or inhibiting its function. In contrast, substances that bind directly to an enzyme's active site or the binding site of the endogenous ligand of a receptor are called orthosteric regulators or modulators. The site to which the effector binds is termed the ''allosteric site'' or ''regulatory site''. Allosteric sites allow effectors to bind to the protein, often resulting in a conformational change and/or a change in protein dynamics. Effectors that enhance the protein's activity are referred to as ''allosteric activators'', whereas those that decrease the protein's activity are called ''allosteric inhibitors''. Allosteric regulations are a natural example of control loops, such as feedback from downs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-throughput Screening
High-throughput screening (HTS) is a method for scientific discovery especially used in drug discovery and relevant to the fields of biology, materials science and chemistry. Using robotics, data processing/control software, liquid handling devices, and sensitive detectors, high-throughput screening allows a researcher to quickly conduct millions of chemical, genetic, or pharmacological tests. Through this process one can quickly recognize active compounds, antibodies, or genes that modulate a particular biomolecular pathway. The results of these experiments provide starting points for drug design and for understanding the noninteraction or role of a particular location. Assay plate preparation The key labware or testing vessel of HTS is the microtiter plate, which is a small container, usually disposable and made of plastic, that features a grid of small, open divots called ''wells''. In general, microplates for HTS have either 96, 192, 384, 1536, 3456 or 6144 wells. These are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |