|
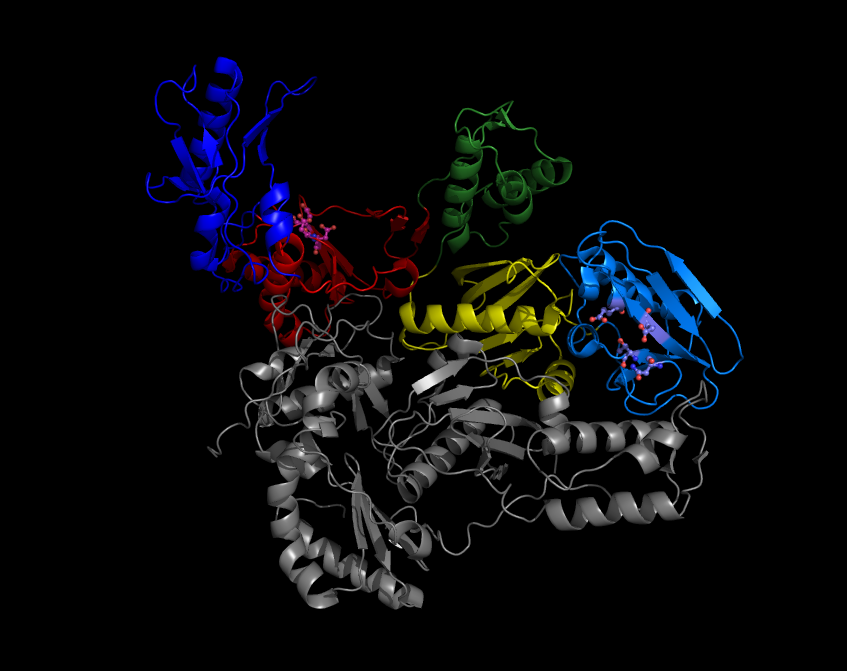
Azvudine
Azvudine is an antiviral drug which acts as a reverse transcriptase inhibitor. It was discovered for the treatment of hepatitis C and has since been investigated for use against other viral diseases such as AIDS and COVID-19, for which it was granted conditional approval in China. Azvudine was first discovered in 2007.Chang J, Bao X, Wang Q, Guo X, Wang W, Qi XPreparation of 2′-fluoro-4′-substituted nucleoside analogs as antiviral agents.20070807. Chinese Patent Application No: CN 2007-10137548. Chinese Patent No: CN 101177442A, 20080514. It costs 350 Chinese yuan per 7 days for COVID, . Medical uses In July 2021, azvudine became conditionally approved in China for the following indication: "to treat high-viral-load cases of HIV-1, in combination with a nucleoside reverse-transcriptase inhibitor and a non-nucleoside reverse-transcriptase inhibitor". The approval text describes it as a dual reverse transcriptase and Vif inhibitor. In July 2022, azvudine received emergenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reverse Transcriptase Inhibitor
Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses. Mechanism of action When HIV infects a cell, reverse transcriptase copies the viral single stranded RNA genome into a double-stranded viral DNA. The viral DNA is then integrated into the host chromosomal DNA, which then allows host cellular processes, such as transcription and translation, to reproduce the virus. RTIs block reverse transcriptase's enzymatic function and prevent completion of synthesis of the double-stranded viral DNA, thus preventing HIV from multiplying. A similar process occurs with other types of viruses. The hepatitis B virus, for example, carries its genetic material in the form of DNA, and employs an RNA-dependent DNA polymerase to replicate. Some of the same compounds used as RTIs ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP3A
Cytochrome P450, family 3, subfamily A, also known as CYP3A, is a human gene locus. A homologous locus is found in mice. These genes encode monooxygenases which catalyze many reactions involved in the synthesis of cholesterol, steroids and other lipids as well as drug metabolism. The CYP3A locus includes all the known members of the 3A subfamily of the cytochrome P450 superfamily of genes. The CYP3A cluster consists of four genes: * CYP3A4, * CYP3A5, * CYP3A7, and * CYP3A43. The region also contains four pseudogenes: * , * , * , and * . as well as several extra exons which may or may not be included in transcripts produced from this region. Previously another CYP3A member, CYP3A3, was thought to exist; however, it is now thought that this sequence represents a transcript variant of CYP3A4. Structure Structurally, the key to the CYP3A enzyme’s large range of activity is the heme cofactor and the P450 protein fold, an oxidation reaction through molecular oxygen and NADPH. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medication Package Insert
A package insert is a document included in the package of a medication that provides information about that drug and its use. For prescription medications, the insert is technical, providing information for medical professionals about how to prescribe the drug. Package inserts for prescription drugs often include a separate document called a "patient package insert" with information written in plain language intended for the end-user—the person who will take the drug or give the drug to another person, such as a minor. Inserts for over-the-counter medications are also written plainly. In the United States, labelling for the healthcare practitioner is called "Prescribing Information" (PI), and labelling for patients and/or caregivers includes "Medication Guides", "Patient Package Inserts", and "Instructions for Use". In Europe, the technical document is called the "summary of product characteristics" (SmPC), and the document for end-users is called the "patient information leaf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiviral Drugs
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are a class of antimicrobials, a larger group which also includes antibiotic (also termed antibacterial), antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from virucides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural virucides are produced by some plants such as eucalyptus and Australian tea trees. Medical uses Most of the antiviral drugs now available are designed to help deal with HIV, herpes viruses, the hepatitis B and C viruses, and influenza A and B viruses. Viruses use the host's cells to replicate and this makes i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Healthcare Security Administration
The Nation Healthcare Security Administration (), abbreviated as NHSA () is a deputy-ministerial-level government agency directly under the State Council of the People's Republic of China. The current director is Zhang Ke. History The administration was created in 2018 as part of the deepening the reform of the Party and state institutions The deepening the reform of the Party and state institutions ( zh, , p=Shēnhuà dǎng hé guójiā jīgòu gǎigé, s=深化党和国家机构改革) was a large-scale reform of the institutions of the Chinese Communist Party (CCP) and the Peopl .... Functions NHSA primarily oversees the state-backed China Healthcare Security including general health insurance plan, maternity insurance, and medical aid programs. In doing that, it oversees the operation of the insurance fund, responsible for centralized purchasing of drugs and medical supplies. It is also responsible for the public-sector healthcare reform. Leadership Directors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First-in-class Medication
A first-in-class medication is a prototype drug that uses a "new and unique mechanism of action" to treat a particular medical condition. While the Food and Drug Administration's Center for Drug Evaluation and Research tracks first-in-class medications and reports on them annually, first-in-class is not considered a regulatory category. Although many first-in-class medications qualify as breakthrough therapies, Regenerative Medicine Advanced Therapies and/or orphan drugs, first-in-class status itself has no regulatory effect. Examples Controversy Safety By definition, a first-in-class drug does not have the safety evidence from analogous products that not-first-in-class drugs would have. However, a study investigating recalls and warnings in relation to first-in-class drugs approved between 1997 and 2012 by Health Canada has found that first-in-class drugs actually have a more favourable benefit-to-harm ratio. Economics First-in-class drugs are often seen as commerc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Medical Products Administration
The National Medical Products Administration (NMPA; ) is a national bureau responsible for drug supervision under the State Council of China and is managed by the State Administration for Market Regulation. History The agency had multiple former names, including China Food and Drug Administration and State Food and Drug Administration. The National Medical Products Administration was founded on the basis of the former State Food and Drug Administration (SFDA). In March 2013, the former regulatory body was rebranded and restructured as the China Food and Drug Administration, elevating it to a ministerial-level agency. In 2018, as part of China's 2018 government administration overhaul, the name was changed to 'National Medical Products Administration' and merged into the newly created State Administration for Market Regulation. The headquarters are in Xicheng, Beijing. In its first incarnation as the CFDA, the NMPA replaced a large group of overlapping regulators with an ent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reverse Transcriptase
A reverse transcriptase (RT) is an enzyme used to convert RNA genome to DNA, a process termed reverse transcription. Reverse transcriptases are used by viruses such as HIV and hepatitis B to replicate their genomes, by retrotransposon mobile genetic elements to proliferate within the host genome, and by eukaryotic cells to extend the telomeres at the ends of their linear chromosomes. The process does not violate the flows of genetic information as described by the classical central dogma, but rather expands it to include transfers of information from RNA to DNA. Retroviral RT has three sequential biochemical activities: RNA-dependent DNA polymerase activity, ribonuclease H (RNase H), and DNA-dependent DNA polymerase activity. Collectively, these activities enable the enzyme to convert single-stranded RNA into double-stranded cDNA. In retroviruses and retrotransposons, this cDNA can then integrate into the host genome, from which new RNA copies can be made via host-cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zhengzhou University
Zhengzhou University (ZZU; ) is a provincial public university in Zhengzhou, Henan, China. It is affiliated with the Province of Henan. The university is part of Project 211 and the Double First-Class Construction. Zhengzhou University is ranked 511th in the QS World University Rankings 2025, recognizing it as one of the top global universities. Zhengzhou University is the largest university in China in terms of number of students (around 73,000 students). The campus size is . History Zhengzhou University is the first comprehensive university established by the State Council of Chinese Government in 1954. According to the State Council, a group of faculty members from Shandong University, Peking University, Jilin University and Northeast University moved to Zhengzhou. The Zhengzhou University was established by these professors in 1954. Henan Medical University dates back to the medical education in National Fifth Sun Yat-Sen University built in 1928 (the predecessor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Body Mass
Human body weight is a person's mass or weight. Strictly speaking, body weight is the measurement of mass without items located on the person. Practically though, body weight may be measured with clothes on, but without shoes or heavy accessories such as mobile phones and wallets, and using manual or digital weighing scales. Excess or reduced body weight is regarded as an indicator of determining a person's health, with body volume measurement providing an extra dimension by calculating the distribution of body weight. Average adult human weight varies by continent, from about in Asia and Africa to about in North America, with men on average weighing more than women. Estimation in children There are a number of methods to estimate weight in children for circumstances (such as emergencies) when actual weight cannot be measured. Most involve a parent or health care provider guessing the child's weight through weight-estimation formulas. These formulas base their findings on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
No-observed-adverse-effect Level
The no-observed-adverse-effect level (NOAEL) denotes the level of exposure of an organism, found by experiment or observation, at which there is no biologically or statistically significant increase in the frequency or severity of any adverse effects of the tested protocol. In drug development, the NOAEL of a new drug is assessed in laboratory animals, such as mice, prior to initiation of human trials in order to establish a safe clinical starting dose in humans. The OECD publishes guidelines for Preclinical Safety Assessments, in order to help scientists discover the NOAEL. Synopsis Some adverse effects in the exposed population when compared to its appropriate control might include alteration of morphology, functional capacity, growth, development or life span. The NOAEL is determined or proposed by qualified personnel, often a pharmacologist or a toxicologist. The NOAEL could be defined as "the highest experimental point that is without adverse effect," meaning that under ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ames Test
The Ames test is a widely employed method that uses bacteria to test whether a given chemical can cause mutations in the DNA of the test organism. More formally, it is a bioassay, biological assay to assess the mutagenic potential of chemical compounds. A positive test indicates that the chemical is mutagenic and therefore may act as a carcinogen, because cancer is often linked to mutation. The test serves as a quick and convenient assay to estimate the carcinogenic potential of a compound because standard carcinogen assays on mice and rats are time-consuming (taking two to three years to complete) and expensive. However, false-positives and false-negatives are known. The procedure was described in a series of papers in the early 1970s by Bruce Ames and his group at the University of California, Berkeley. General procedure The Ames test uses several strains of the bacterium ''Salmonella typhimurium'' that carry mutations in genes involved in histidine synthesis. These strains a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |