|
Arsenic Biochemistry
Arsenic biochemistry is the set of biochemical processes that can use arsenic or its compounds, such as arsenate. Arsenic is a moderately abundant element in Earth's crust, and although many arsenic compounds are often considered highly toxic to most life, a wide variety of organoarsenic compounds are produced biologically and various organic and inorganic arsenic compounds are metabolized by numerous organisms. This pattern is general for other related elements, including selenium, which can exhibit both beneficial and deleterious effects. Arsenic biochemistry has become topical since many toxic arsenic compounds are found in some aquifers, potentially affecting many millions of people via biochemical processes.Elke Dopp, Andrew D. Kligerman and Roland A. Diaz-Bone Organoarsenicals. Uptake, Metabolism, and Toxicity 2010, Royal Society of Chemistry. . Sources of arsenic Organoarsenic compounds in nature The evidence that arsenic may be a beneficial nutrient at trace levels belo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
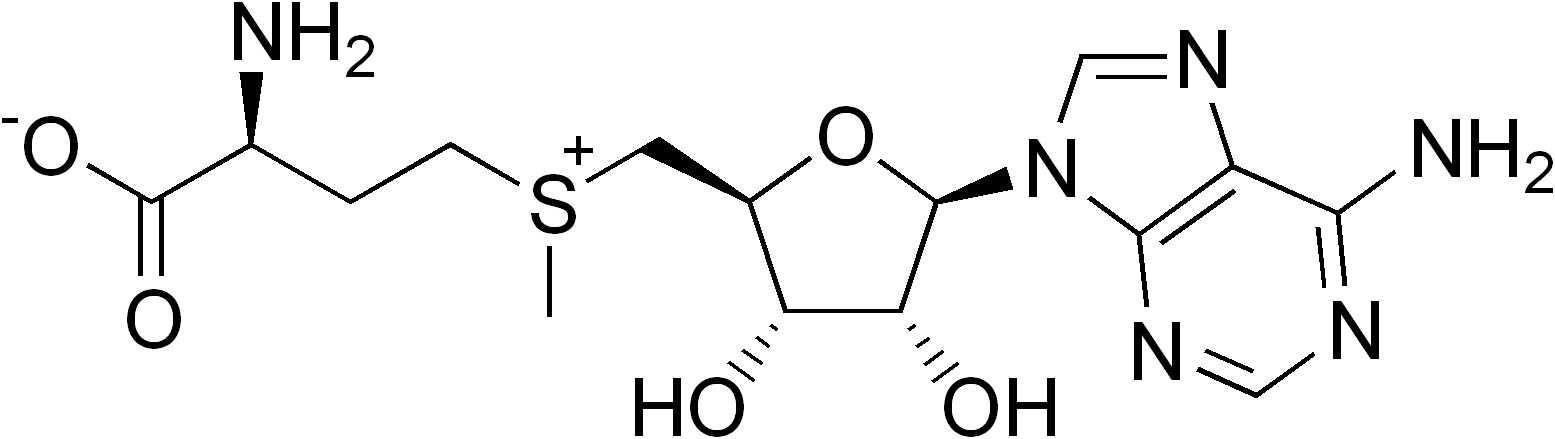
S-Adenosyl Methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cacodylic Acid
Cacodylic acid is an organoarsenic compound with the formula (CH3)2 AsO2H. With the formula R2As(O)OH, it is the simplest of the arsinic acids. It is a colorless solid that is soluble in water. Neutralization of cacodylic acid with base gives cacodylate salts, e.g. sodium cacodylate. They are potent herbicides. Cacodylic acid/sodium cacodylate is a buffering agent in the preparation and fixation of biological samples for electron microscopy and in protein crystallography. History In the 18th century it was found that combining arsenic trioxide () and four equivalents of potassium acetate () gives a product called " Cadet's fuming liquid" which contains cacodyl oxide, and cacodyl, . Early research into "cacodyls" was reported by Robert Bunsen at the University of Marburg. Bunsen said of the compounds, "The smell of this body produces instantaneous tingling of the hands and feet, and even giddiness and insensibility... It is remarkable that when one is exposed to the sme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Roxarsone
Roxarsone is an organoarsenic compound that has been used in poultry production as a feed additive to increase weight gain and improve feed efficiency, and as a coccidiostat. As of June 2011, it was approved for chicken feed in the United States, Canada, Australia, and 12 other countries. The drug was also approved in the United States and elsewhere for use in pigs. Its use in the United States was voluntarily ended by the manufacturers in June 2011 and has been illegal since 2013. Its use was immediately suspended in Malaysia. It was banned in Canada in August 2011. In Australia, its use in chicken feed was discontinued in 2012. Roxarsone has been banned in the European Union since 1999. Production and applications Roxarsone is a derivative of phenylarsonic acid (C6H5As(O)(OH)2). It was first reported in a 1923 British patent that described the nitration and diazotization of arsanilic acid. When blended with calcite powder, it is used in poultry feed premixes in some parts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic Trioxide
Arsenic trioxide is an inorganic compound with the formula . As an industrial chemical, its major uses include the manufacture of wood preservatives, pesticides, and glass. It is sold under the brand name Trisenox among others when used as a medication to treat a type of cancer known as acute promyelocytic leukemia. For this use it is given by injection into a vein. Common side effects include vomiting, diarrhea, swelling, shortness of breath, and headaches. Severe side effects may include APL differentiation syndrome and heart problems. Use during pregnancy or breastfeeding may harm the baby. Its mechanism in treating cancer is not entirely clear. Arsenic trioxide was approved for medical use in the United States in 2000. It is on the World Health Organization's List of Essential Medicines. Approximately 50,000 tonnes are produced a year. Due to its toxicity, a number of countries have regulations around its manufacture and sale. Uses Medical Arsenic trioxide is indicat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenide
In chemistry, an arsenide is a compound of arsenic with a less electronegative element or elements. Many metals form binary compounds containing arsenic, and these are called arsenides. They exist with many Stoichiometry, stoichiometries, and in this respect arsenides are similar to phosphides. Alkali metal and alkaline earth arsenides The group 1 alkali metals and the group 2, alkaline earth metals, form arsenides with isolated arsenic atoms. They form upon heating arsenic powder with excess sodium gives sodium arsenide (Na3As). The structure of Na3As is complex with unusually short Na–Na distances of 328–330 pm which are shorter than in sodium metal. This short distance indicates the complex bonding in these simple phases, i.e. they are not simply salts of As3− anion, for example. The compound LiAs, has a metallic lustre and electrical conductivity indicating some metallic bonding. These compounds are mainly of academic interest. For example, "sodium arsenide" is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Roasting (metallurgy)
Roasting is a process of heating an ore to under flowing air. It is a step in the processing of certain ores. More specifically, roasting is often a metallurgical process involving gas–solid reactions at elevated temperatures with the goal of purifying the metal component(s). Often before roasting, the ore has already been partially purified, e.g. by froth flotation. The concentrate is mixed with other materials to facilitate the process. The technology is useful in making certain ores usable but it can also be a serious source of air pollution. Roasting consists of thermal gas–solid reactions, which can include oxidation, reduction, chlorination, sulfation, and pyrohydrolysis. In roasting, the ore or ore concentrate is treated with very hot air. This process is generally applied to sulfide minerals. During roasting, the sulfide is converted to an oxide, and sulfur is released as sulfur dioxide, a gas. For the ores Cu2S ( chalcocite) and ZnS (sphalerite), balance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfide Mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide () as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenide mineral, selenides, the telluride mineral, tellurides, the arsenide mineral, arsenides, the antimonide mineral, antimonides, the bismuthinides, the sulfarsenides and the sulfosalts.http://www.minerals.net/mineral/sort-met.hod/group/sulfgrp.htm Minerals.net Dana Classification, SulfidesKlein, Cornelis and Cornelius S. Hurlbut, Jr., 1986, ''Manual of Mineralogy'', Wiley, 20th ed., pp 269-293 Sulfide minerals are inorganic compounds. Minerals Common or important examples include: * Acanthite *Chalcocite *Bornite *Galena *Sphalerite *Chalcopyrite *Pyrrhotite *Millerite *Pentlandite *Covellite *Cinnabar *Realgar *Orpiment *Stibnite *Pyrite *Marcasite *Molybdenite Sulfarsenides: *Cobaltite *Arsenopyrite *Gersdorffite Sulfosalts: *Pyrargyrite *Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GFAJ-1
GFAJ-1 is a strain of rod-shaped bacteria in the family Halomonadaceae. It is an extremophile that was isolated from the hypersaline and alkaline Mono Lake in eastern California by geobiologist Felisa Wolfe-Simon, a NASA research fellow in residence at the US Geological Survey. In a 2010 ''Science'' journal publication, the authors claimed that the microbe, when starved of phosphorus, is capable of substituting arsenic for a small percentage of its phosphorus to sustain its growth. Immediately after publication, other microbiologists and biochemists expressed doubt about this claim, which was robustly criticized in the scientific community. Subsequent independent studies published in 2012 found no detectable arsenate in the DNA of GFAJ-1, refuted the claim, and demonstrated that GFAJ-1 is simply an arsenate-resistant, phosphate-dependent organism. Discovery The GFAJ-1 bacterium was discovered by geomicrobiologist Felisa Wolfe-Simon, a NASA astrobiology fellow in residence at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Science (journal)
''Science'' is the peer review, peer-reviewed academic journal of the American Association for the Advancement of Science (AAAS) and one of the world's top academic journals. It was first published in 1880, is currently circulated weekly and has a subscriber base of around 130,000. Because institutional subscriptions and online access serve a larger audience, its estimated readership is over 400,000 people. ''Science'' is based in Washington, D.C., United States, with a second office in Cambridge, UK. Contents The major focus of the journal is publishing important original scientific research and research reviews, but ''Science'' also publishes science-related news, opinions on science policy and other matters of interest to scientists and others who are concerned with the wide implications of science and technology. Unlike most scientific journals, which focus on a specific field, ''Science'' and its rival ''Nature (journal), Nature'' cover the full range of List of academ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biota (ecology)
A biome () is a distinct geographical region with specific climate, vegetation, and animal life. It consists of a biological community that has formed in response to its physical environment and regional climate. In 1935, Tansley added the climatic and soil aspects to the idea, calling it '' ecosystem''. The International Biological Program (1964–74) projects popularized the concept of biome. However, in some contexts, the term ''biome'' is used in a different manner. In German literature, particularly in the Walter terminology, the term is used similarly as '' biotope'' (a concrete geographical unit), while the biome definition used in this article is used as an international, non-regional, terminology—irrespectively of the continent in which an area is present, it takes the same biome name—and corresponds to his "zonobiome", "orobiome" and "pedobiome" (biomes determined by climate zone, altitude or soil). In the Brazilian literature, the term ''biome'' is sometim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paris Green
Paris green (copper(II) acetate triarsenite or copper(II) acetoarsenite) is an arsenic-based organic pigment. As a green pigment it is also known as Mitis green, Schweinfurt green, Sattler green, emerald, Vienna green, Emperor green or Mountain green. It is a highly toxic emerald-green crystalline powder that has been used as a rodenticide and insecticide, and also as a pigment. It was manufactured in 1814 to be a pigment to make a vibrant green paint, and was used by many notable painters in the 19th century. The color of Paris green is said to range from a pale blue green when very finely ground, to a deeper green when coarsely ground. Due to the presence of arsenic, the pigment is extremely toxic. In paintings, the color can degrade quickly. Preparation and structure Paris green may be prepared by combining copper(II) acetate and arsenic trioxide."H.Wayne Richardson, "Copper Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. The str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally occurring form, , is a component of the ribonucleotides from which RNA is built, and so this compound is necessary for coding, decoding, regulation and expression of genes. It has a structural analog, deoxyribose, which is a similarly essential component of DNA. is an unnatural sugar that was first prepared by Emil Fischer and Oscar Piloty in 1891. It was not until 1909 that Phoebus Levene and Walter Jacobs recognised that was a natural product, the enantiomer of Fischer and Piloty's product, and an essential component of nucleic acids. Fischer chose the name "ribose" as it is a partial rearrangement of the name of another sugar, arabinose, of which ribose is an epimer at the 2' carbon; both names also relate to gum arabic, from which arabinose was first isolated and from which they prepared . Like most sugars, ribo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |