|
Armour Research Foundation
IIT Research Institute (IITRI),Greenbaum & Wheeler (1967), cover sheet (technical paper).McCormac; et al. (1967), p. i (book)."IITRI" (or "iiTRi") is used on cover sheets of technical paper documents in prior decades. also known historically and interchangeably as IIT Research Center,"IIT Research Center" appears under document title heading labels used on cover sheets of technical paper documents in prior decades. This can also appear together on the same sheet with the background abbreviation "IITRI" (or "iiTRi")."IIT Research Center" appears on one of the recipient address pages (p. 23) in Rome, New York on the lower right side of the Gama; et al. (2004) reference. is a high-technology scientific research organization and applied research laboratory located in Chicago, Illinois. Previously known as the Armour Research Foundation,Actual letter stationery in the period notes in its header "IIT Research Institute" as the primary letterhead first line. The second line underneath ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marvin Camras
Marvin Camras (January 1, 1916 – June 23, 1995) was an electrical engineer and inventor who was widely influential in the field of magnetic recording. Camras built his first recording device, a wire recorder, in the 1930s for a cousin who was an aspiring opera singer named Willy. He also built Willy a telephone, because he could not afford one, at the age of 8. Shortly afterwards he discovered that using magnetic tape made the process of splicing and storing recordings easier. Camras's work attracted the notice of his professors at what became Illinois Institute of Technology (IIT) and was offered a position at Armour Research Foundation (which merged with Lewis Institute in 1940 to become IIT) to develop his work. Before and during World War II Camras' wire recorders were used by the armed forces to train pilots. They were also used for disinformation purposes: battle sounds were recorded and amplified and the recordings placed where the D-Day invasion was not going to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Centers For Disease Control And Prevention
The Centers for Disease Control and Prevention (CDC) is the National public health institutes, national public health agency of the United States. It is a Federal agencies of the United States, United States federal agency under the United States Department of Health and Human Services, Department of Health and Human Services (HHS), and is headquartered in Atlanta, Georgia. The CDC's current nominee for director is Susan Monarez. She became acting director on January 23, 2025, but stepped down on March 24, 2025 when nominated for the director position. On May 14, 2025, Robert F. Kennedy Jr. stated that lawyer Matthew Buzzelli is acting CDC director. However, the CDC web site does not state the acting director's name. The agency's main goal is the protection of public health and safety through the control and prevention of disease, injury, and disability in the US and worldwide. The CDC focuses national attention on developing and applying disease control and prevention. It e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Telemetry
Telemetry is the in situ collection of measurements or other data at remote points and their automatic transmission to receiving equipment (telecommunication) for monitoring. The word is derived from the Greek roots ''tele'', 'far off', and ''metron'', 'measure'. Systems that need external instructions and data to operate require the counterpart of telemetry: telecommand. Although the term commonly refers to wireless data transfer mechanisms (e.g., using radio, ultrasonic, or infrared systems), it also encompasses data transferred over other media such as a telephone or computer network, optical link or other wired communications like power line carriers. Many modern telemetry systems take advantage of the low cost and ubiquity of GSM networks by using SMS to receive and transmit telemetry data. A ''telemeter'' is a physical device used in telemetry. It consists of a sensor, a transmission path, and a display, recording, or control device. Electronic devices are widely u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Endpoint
Clinical endpoints or clinical outcomes are outcome measures referring to occurrence of disease, symptom, sign or laboratory abnormality constituting a target outcome in clinical research trials. The term may also refer to any disease or sign that strongly motivates withdrawal of an individual or entity from the trial, then often termed a ''humane (clinical) endpoint''. The primary endpoint of a clinical trial is the endpoint for which the trial is powered. Secondary endpoints are additional endpoints, preferably also pre-specified, for which the trial may not be powered. Surrogate endpoints are trial endpoints that have outcomes that substitute for a clinical endpoint, often because studying the clinical endpoint is difficult, for example using an increase in blood pressure as a surrogate for death by cardiovascular disease, where strong evidence of a causal link exists. Scope In a general sense, a clinical endpoint is included in the entities of interest in a trial. The re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacokinetics
Pharmacokinetics (from Ancient Greek ''pharmakon'' "drug" and ''kinetikos'' "moving, putting in motion"; see chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to describing how the body affects a specific substance after administration. The substances of interest include any chemical xenobiotic such as pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is based on mathematical modeling that places great emphasis on the relationship between drug plasma concentration and the time elapsed since the drug's administration. Pharmacokinetics is the study of how an organism affects the drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C). However, the agency also enforces other laws, notably Section 361 of the Public Health Service Act as well as associated regulations. Much of this regulatory-enforcement work is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Discovery
In the fields of medicine, biotechnology, and pharmacology, drug discovery is the process by which new candidate medications are discovered. Historically, drugs were discovered by identifying the active ingredient from traditional remedies or by serendipitous discovery, as with penicillin. More recently, chemical libraries of synthetic small molecules, natural products, or extracts were screened in intact cells or whole organisms to identify substances that had a desirable therapeutic effect in a process known as classical pharmacology. After sequencing of the human genome allowed rapid cloning and synthesis of large quantities of purified proteins, it has become common practice to use high throughput screening of large compounds libraries against isolated biological targets which are hypothesized to be disease-modifying in a process known as reverse pharmacology. Hits from these screens are then tested in cells and then in animals for efficacy. Modern drug discovery i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Investigational New Drug
The United States Food and Drug Administration's Investigational New Drug (IND) program is the means by which a pharmaceutical industry, pharmaceutical company obtains permission to start human clinical trials and to ship an experimental drug interstate commerce, across state lines (usually to clinical investigators) before a marketing application for the drug has been approved. Regulations are primarily at . Similar procedures are followed in the European Union, Japan, and Canada due to regulatory harmonization efforts by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, International Council for Harmonisation. Types * Research or investigator INDs are non-commercial INDs filed by researchers to study an unapproved drug or to study an approved drug for a new indication or in a new patient population. * Expanded access, Emergency Use INDs, also called compassionate use or single-patient INDs, are filed for emergency use of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
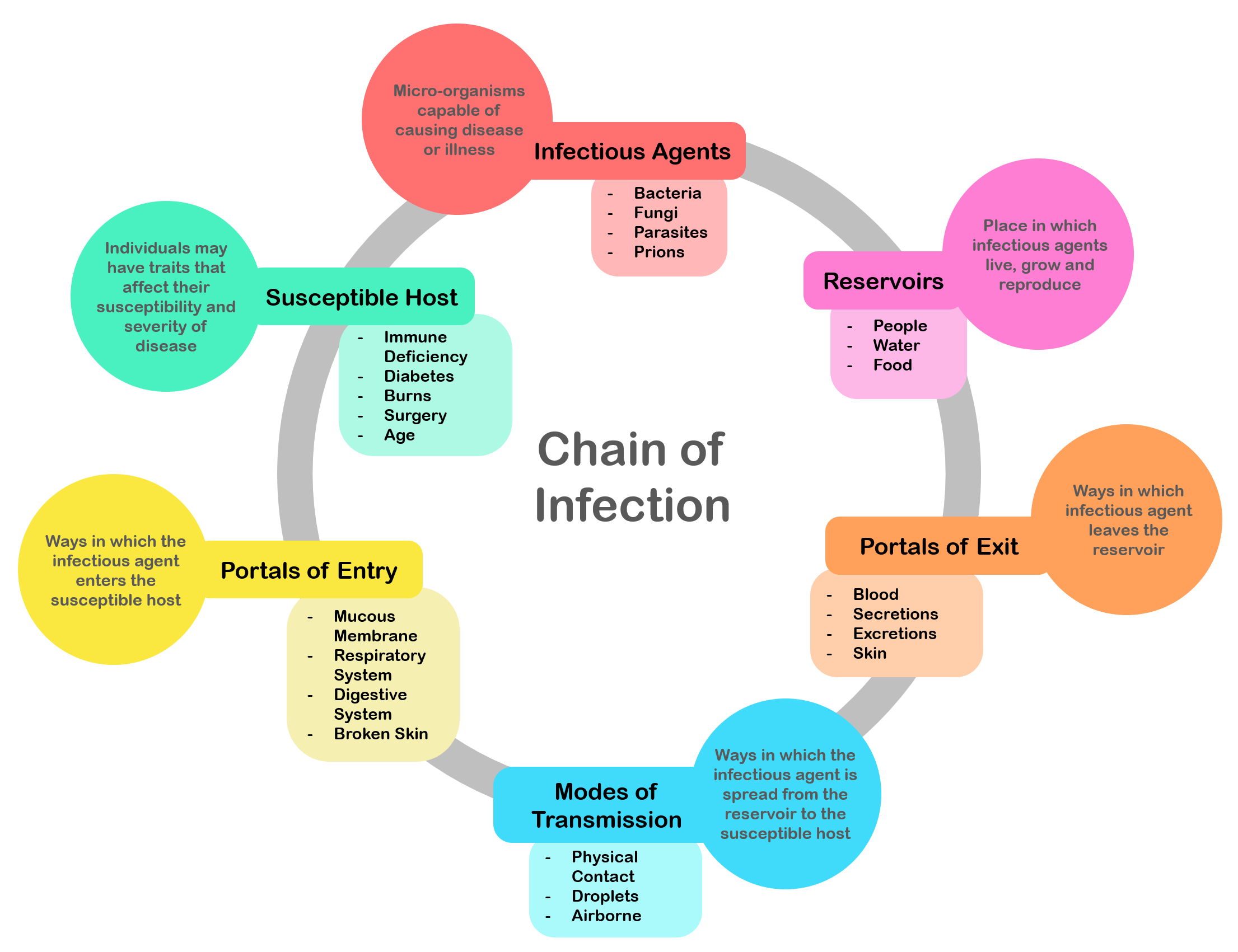
Infectious Disease
An infection is the invasion of tissue (biology), tissues by pathogens, their multiplication, and the reaction of host (biology), host tissues to the infectious agent and the toxins they produce. An infectious disease, also known as a transmissible disease or communicable disease, is an Disease#Terminology, illness resulting from an infection. Infections can be caused by a wide range of pathogens, most prominently pathogenic bacteria, bacteria and viruses. Hosts can fight infections using their immune systems. Mammalian hosts react to infections with an Innate immune system, innate response, often involving inflammation, followed by an Adaptive immune system, adaptive response. Treatment for infections depends on the type of pathogen involved. Common medications include: * Antibiotics for bacterial infections. * Antivirals for viral infections. * Antifungals for fungal infections. * Antiprotozoals for protozoan infections. * Antihelminthics for infections caused by parasi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible Signs and symptoms of cancer, signs and symptoms include a lump, abnormal bleeding, prolonged cough, unexplained weight loss, and a change in defecation, bowel movements. While these symptoms may indicate cancer, they can also have other causes. List of cancer types, Over 100 types of cancers affect humans. Tobacco use is the cause of about 22% of cancer deaths. Another 10% are due to obesity, poor Diet (nutrition), diet, sedentary lifestyle, lack of physical activity or Alcohol abuse, excessive alcohol consumption. Other factors include certain infections, exposure to ionizing radiation, and environmental pollutants. infectious causes of cancer, Infection with specific viruses, bacteria and parasites is an environmental factor cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Contract Research Organization
In the life sciences, a contract research organization (CRO) is a company that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. A CRO may provide such services as biopharmaceutical development, biological assay development, commercialization, clinical development, clinical trials management, pharmacovigilance, outcomes research, and real world evidence. CROs are designed to reduce costs for companies developing new medicines and drugs in niche markets. They aim to simplify entry into drug markets, and simplify development, as the need for large pharmaceutical companies to do everything ‘in house’ is now redundant. CROs also support foundations, research institutions, and universities, in addition to governmental organizations (such as the NIH, EMA, etc.). Many CROs specifically provide clinical-study and clinical-trial support for drugs and/or medical devices. However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |