|
Argon Compound
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO. Argon ionises at 15.76 eV, which is higher than hydrogen, but lower than helium, neon or fluorine. Molecules containing argon can be van der Waals molecules held together very weakly by London dispersion forces. Ionic molecules can be bound by charge induced dipole interactions. With gold atoms there can be some covalent interaction. Several boron-argon bonds with significant covalent interactions have been also reported. Experimental methods used to study argon compounds have included inert gas matrices, infrared spectroscopy to study stretching a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust. Nearly all argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas. The name "argon" is derived from the Greek word , neuter singular form of meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The complete oc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Effective Core Potential
In physics, a pseudopotential or effective potential is used as an approximation for the simplified description of complex systems. Applications include atomic physics and neutron scattering. The pseudopotential approximation was first introduced by Hans Hellmann in 1934. Atomic physics The pseudopotential is an attempt to replace the complicated effects of the motion of the core (i.e. non- valence) electrons of an atom and its nucleus with an effective potential, or pseudopotential, so that the Schrödinger equation contains a modified effective potential term instead of the Coulombic potential term for core electrons normally found in the Schrödinger equation. The pseudopotential is an effective potential constructed to replace the atomic all-electron potential (full-potential) such that core states are eliminated ''and'' the valence electrons are described by pseudo-wavefunctions with significantly fewer nodes. This allows the pseudo-wavefunctions to be described with fa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Icosahedral
In geometry, an icosahedron ( or ) is a polyhedron with 20 faces. The name comes . The plural can be either "icosahedra" () or "icosahedrons". There are infinitely many non- similar shapes of icosahedra, some of them being more symmetrical than others. The best known is the (convex, non- stellated) regular icosahedron—one of the Platonic solids—whose faces are 20 equilateral triangles. Regular icosahedra There are two objects, one convex and one nonconvex, that can both be called regular icosahedra. Each has 30 edges and 20 equilateral triangle faces with five meeting at each of its twelve vertices. Both have icosahedral symmetry. The term "regular icosahedron" generally refers to the convex variety, while the nonconvex form is called a ''great icosahedron''. Convex regular icosahedron The convex regular icosahedron is usually referred to simply as the ''regular icosahedron'', one of the five regular Platonic solids, and is represented by its Schläfli symbol , containi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ångström
The angstrom (; ) is a unit of length equal to m; that is, one ten- billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). It was originally spelled with Swedish letters, as Ångström and later as ångström (). The latter spelling is still listed in some dictionaries, but is now rare in English texts. Some popular US dictionaries list only the spelling ''angstrom''. The unit's symbol is Å, which is a letter of the Swedish alphabet, regardless of how the unit is spelled. However, "A" or "A.U." may be used in less formal contexts or typographically limited media. The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals, wavelengths of electromagnetic radiation, and dimensions of integrated circuit p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
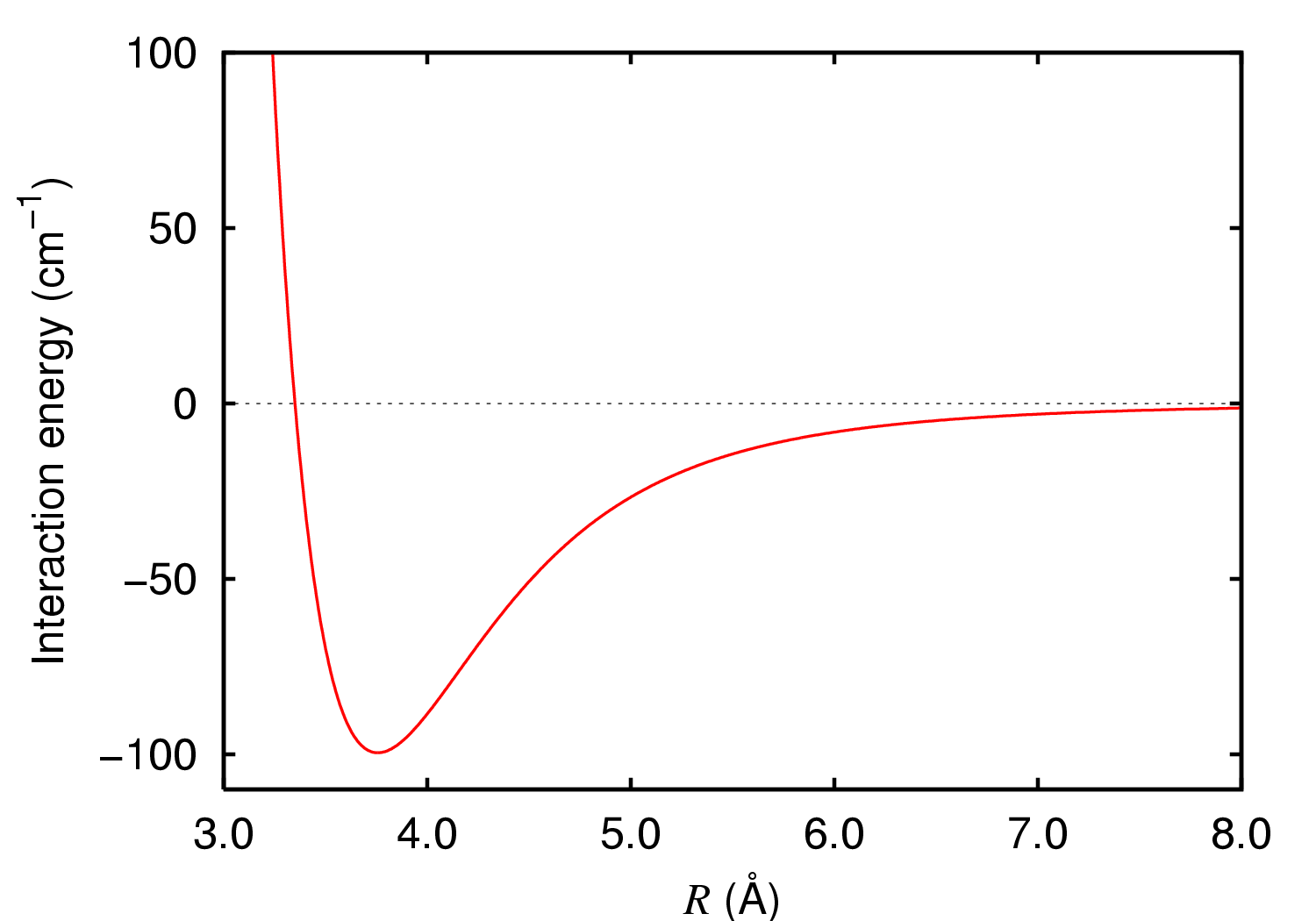
Diargon
Diargon or the argon dimer is a molecule containing two argon atoms. Normally, this is only very weakly bound together by van der Waals forces (a van der Waals molecule). However, in an excited state, or ionised state, the two atoms can be more tightly bound together, with significant spectral features. At cryogenic temperatures, argon gas can have a few percent of diargon molecules. Theory Two argon atoms are attracted together by van der Waals forces when far from each other. When they are close, electrostatic forces repel them. There is a balance point where the van der Waals force matches the opposing repelling force, where energy is at a minimum, represented as the trough in the graph of interaction energy versus distance. This distance is the ground state of the unexcited argon dimer. In a vibrating molecule, the distance between the atoms bounces backwards and forwards from one side of the trough to the other. Faster vibrations will force the state up to higher levels i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Column Density
The area density (also known as areal density, surface density, superficial density, areic density, column density, or density thickness) of a two-dimensional object is calculated as the mass per unit area. The SI derived unit is the "kilogram per square metre" (kg·m−2). In the paper and fabric industries, it is called grammage and is expressed in grams per square meter (g/m2); for paper in particular, it may be expressed as pounds per ream of standard sizes ("basis ream"). A related '' area number density'' can be defined by replacing mass by number of particles or other countable quantity, with resulting units of m−2. Formulation Area density can be calculated as: \rho_A = \frac or \rho_A = \rho \cdot l, where ' is the average area density, ' is the total mass of the object, ' is the total area of the object, ' is the average density, and ' is the average thickness of the object. Column density A special type of area density is called ''column density'' (also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emission Line
A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. It may result from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected ones of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible. Types of line spectra Spectral lines are the result of interaction between a quantum system (usually atoms, but sometimes molecules or atomic nuclei) and a single photon. When a photon has about the right amount of energy (which is connected to its frequency) to allow a change in the energy state of the system (in the case of an atom this is usually an electron changing orbitals), the photon is absorbed. Then the energy will be spontaneously re-emitted, either as one photon at the same f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crab Nebula
The Crab Nebula (catalogue designations M1, NGC 1952, Taurus A) is a supernova remnant and pulsar wind nebula in the constellation of Taurus (constellation), Taurus. The common name comes from a drawing that somewhat resembled a crab with arms produced by William Parsons, 3rd Earl of Rosse, in 1842 or 1843 using a telescope. The nebula was discovered by English astronomer John Bevis in 1731. It corresponds with SN 1054, a bright supernova observed in 1054 C.E. by Native American, Japanese, and Arabic stargazers; this supernova was also recorded by Chinese astronomy, Chinese astronomers as a Guest star (astronomy), guest star. The nebula was the first astronomical object identified that corresponds with a historically-observed supernova explosion. At an apparent magnitude of 8.4, comparable to that of Titan (moon), Saturn's moon Titan, it is not visible to the naked eye but can be made out using binoculars under favourable conditions. The nebula lies in the Perseus Arm of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrogen Cation
The dihydrogen cation or molecular hydrogen ion is a cation (positive ion) with formula H2^+. It consists of two hydrogen nuclei (protons), each sharing a single electron. It is the simplest molecular ion. The ion can be formed from the ionization of a neutral hydrogen molecule (H2) by electron impact. It is commonly formed in molecular clouds in space by the action of cosmic rays. The dihydrogen cation is of great historical, theoretical, and experimental interest. Historically it is of interest because, having only one electron, the equations of quantum mechanics that describe its structure can be solved approximately in a relatively straightforward way, as long as the motion of the nuclei and relativistic and quantum electrodynamic effects are neglected. The first such solution was derived by Ø. Burrau in 1927, just one year after the wave theory of quantum mechanics was published. The theoretical interest arises because an accurate mathematical description, taking into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Hydrogen
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral hydrogen atom contains a single positively charged proton in the nucleus, and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, a hydrogen atom tends to combine with other atoms in compounds, or with another hydrogen atom to form ordinary (diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms). Atomic spectroscopy shows that there is a discrete infinite set of states in which a hydrogen (or any) atom can exist, contrary to the predictions of classical physics. At ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argonium
Argonium (also called the argon hydride cation, the hydridoargon(1+) ion, or protonated argon; chemical formula ArH+) is a cation combining a proton and an argon atom. It can be made in an electric discharge, and was the first noble gas molecular ion to be found in interstellar space. Properties Argonium is isoelectronic with hydrogen chloride. Its dipole moment is 2.18 D for the ground state. The binding energy is 369 kJ mol−1 (3.9 eV). This is smaller than that of and many other protonated species, but more than that of . Rotationless radiative lifetimes of different vibrational states vary with isotope and become shorter for the more rapid high-energy vibrations: : The force constant in the bond is calculated at 3.88 mdyne/Å2. Reactions *ArH+ + H2 → Ar + *ArH+ + C → Ar + CH+ *ArH+ + N → Ar + NH+ *ArH+ + O → Ar + OH+ *ArH+ + CO → Ar + COH+ But the reverse reaction happens: *Ar + → ArH+ + H. *Ar + → *ArH+ + H2 Ar+ + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |