|
Argentite
In mineralogy, argentite () is cubic silver sulfide (Ag2S), which can only exist at temperatures above , , or . When it cools to ordinary temperatures it turns into its monoclinic polymorph, acanthite. The International Mineralogical Association has decided to reject argentite as a proper mineral. The name "argentite" sometimes also refers to pseudomorphs of argentite: specimens of acanthite which still display some of the outward signs of the cubic crystal form, even though their actual crystal structure is monoclinic due to the lower temperature. This form of acanthite is occasionally found as uneven cubes and octahedra, but more often as dendritic or earthy masses, with a blackish lead-grey color and metallic luster. Argentite belongs to the galena group. Cleavage, which is so prominent a feature in galena, here presents only in traces. The mineral is perfectly sectile and has a shining streak; hardness 2.5, specific gravity is 7.2–7.4. It occurs in mineral veins, and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acanthite
Acanthite is a form of silver sulfide with the chemical formula Ag2S. It crystallizes in the monoclinic system and is the stable form of silver sulfide below . Argentite is the stable form above that temperature. As argentite cools below that temperature its cubic form is distorted to the monoclinic form of acanthite. Below 173 °C acanthite forms directly. Acanthite is the only stable form in normal air temperature. Occurrence Acanthite is a common silver mineral in moderately low-temperature hydrothermal veins and in zones of supergene enrichment. It occurs in association with native silver, pyrargyrite, proustite, polybasite, stephanite, aguilarite, galena, chalcopyrite, sphalerite, calcite and quartz. Acanthite was first described in 1855 for an occurrence in the Jáchymov (Joachimsthal) district, Ore Mountains, Bohemia (today Karlovy Vary Region, Czech Republic). The name is from the Greek "akantha" meaning thorn or arrow, in reference to its crystal shape. Gall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudomorph
In mineralogy, a pseudomorph is a mineral or mineral compound that appears in an atypical form (crystal system), resulting from a substitution process in which the appearance and dimensions remain constant, but the original mineral is replaced by another due to alteration, or chemical substitution, dissolution and refilling, structural changes or incrustation. The name literally means "false form". Terminology for pseudomorphs is "''replacer'' after ''original''", as in ''brookite'' after ''rutile''. The term ''pseudomorphoses'' was initially used by Haüy. Substitution pseudomorph A substitution pseudomorph is a pseudomorph in which one mineral or other material is replaced by another. The original shape of the mineral remains unchanged, but chemical composition, color, hardness, and other properties change to those of the replacing mineral. This happens typically when a mineral of one composition changes by chemical reaction to another of similar composition, retaining t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Minerals
Silver is a chemical element; it has symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. Silver is found in the Earth's crust in the pure, free elemental form ("native silver"), as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. Most silver is produced as a byproduct of copper, gold, lead, and zinc refining. Silver has long been valued as a precious metal. Silver metal is used in many bullion coins, sometimes alongside gold: while it is more abundant than gold, it is much less abundant as a native metal. Its purity is typically measured on a per-mille basis; a 94%-pure alloy is described as "0.940 fine". As one of the seven metals of antiquity, silver has had an enduring role in most human cultures. Other than in currency and as an investment medium (coins and bullion), silver is used in solar panels, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Sulfide
Silver sulfide is an inorganic compound with the formula . A dense black solid, it is the only sulfide of silver. It is useful as a photosensitizer in photography. It constitutes the tarnish that forms over time on silverware and other silver objects. Silver sulfide is insoluble in most solvents, but is degraded by strong acids. Silver sulfide is a network solid made up of silver (electronegativity of 1.98) and sulfur (electronegativity of 2.58) where the bonds have low ionic character (approximately 10%). Formation Silver sulfide naturally occurs as the tarnish on silverware. When combined with silver, hydrogen sulfide gas creates a layer of black silver sulfide patina on the silver, protecting the inner silver from further conversion to silver sulfide. Silver whiskers can form when silver sulfide forms on the surface of silver electrical contacts operating in an atmosphere rich in hydrogen sulfide and high humidity. Such atmospheres can exist in sewage treatment and paper m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sectility
Sectility is the ability of a mineral to be cut into thin pieces with a knife. Minerals that are not sectile will be broken into rougher pieces when cut. Metals and paper are sectile. Sectility can be used to distinguish minerals of similar appearance, and is a form of tenacity. For example, gold is sectile but pyrite ("fool's gold") is not. Sectility in metals is a result of metallic bonding, where valence (bonding) electrons are delocalized and can flow freely between atoms, rather than being shared between specific pairs or groups of atoms, as in covalent bond A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...ing. References Mineralogy {{mineralogy-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jalpaite
Jalpaite is a rare copper silver sulfide mineral with formula Ag3CuS2. It was first described in 1858 for an occurrence in the Leonora Mine, Jalpa, Zacatecas, Mexico and named for the locality. It occurs in low temperature hydrothermal veins at temperatures less than . Associated minerals include acanthite, mckinstryite, galena, sphalerite, pyrite, chalcopyrite, stromeyerite, polybasite, pearceite, tetrahedrite–tennantite Tennantite is a copper arsenic sulfosalt mineral with an ideal formula . Due to variable substitution of the copper by iron and zinc the formula is . It is gray-black, steel-gray, iron-gray or black in color. A closely related mineral, tetrahed ... and native silver. References Sulfide minerals Copper(I) minerals Silver minerals Tetragonal minerals Minerals in space group 141 Minerals described in 1858 {{sulfide-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jalpa, Zacatecas
Jalpa is a town located in the Mexican state of Zacatecas, close to the border with Jalisco and Aguascalientes and about a two hours drive south of the capital city, Zacatecas, Zacatecas, Zacatecas. Jalpa is a colonial-style city, with cobble stone streets, narrow walkways, two main churches: El Señor de Jalpa and La Parroquia de San Antonio, and two plazas. Jalpa was modeled by the French in the 19th century. In the middle of the plaza is a kiosk which remains in good shape today, after hundreds of years. Most houses are painted in bright colors just as in colonial times. The houses are made of adobe and share common walls and most have flat roofs. The original indigenous natives were the Caxcan, Chichimeca and Huichol people. History Origin of the town of Jalpa It was a Nahuatl chiefdom in the 11th century. Jalpa was founded in 1532 by Spanish explorers in search of gold and silver. Jalpa was spelled "Xalpa" by its native Caxcan, Chichimeca, and Huichol people. The first en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nevada
Nevada ( ; ) is a landlocked state in the Western United States. It borders Oregon to the northwest, Idaho to the northeast, California to the west, Arizona to the southeast, and Utah to the east. Nevada is the seventh-most extensive, the 32nd-most populous, and the ninth-least densely populated U.S. state. Nearly three-quarters of Nevada's population live in Clark County, which contains the Las Vegas–Paradise metropolitan area, including three of the state's four largest incorporated cities. Nevada's capital is Carson City. Las Vegas is the largest city in the state. Nevada is officially known as the "Silver State" because of the importance of silver to its history and economy. It is also known as the "Battle Born State" because it achieved statehood during the Civil War (the words "Battle Born" also appear on its state flag); due to the presidency of Abraham Lincoln, the Union benefited immensely from the support of newly awarded statehood by the infusion of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
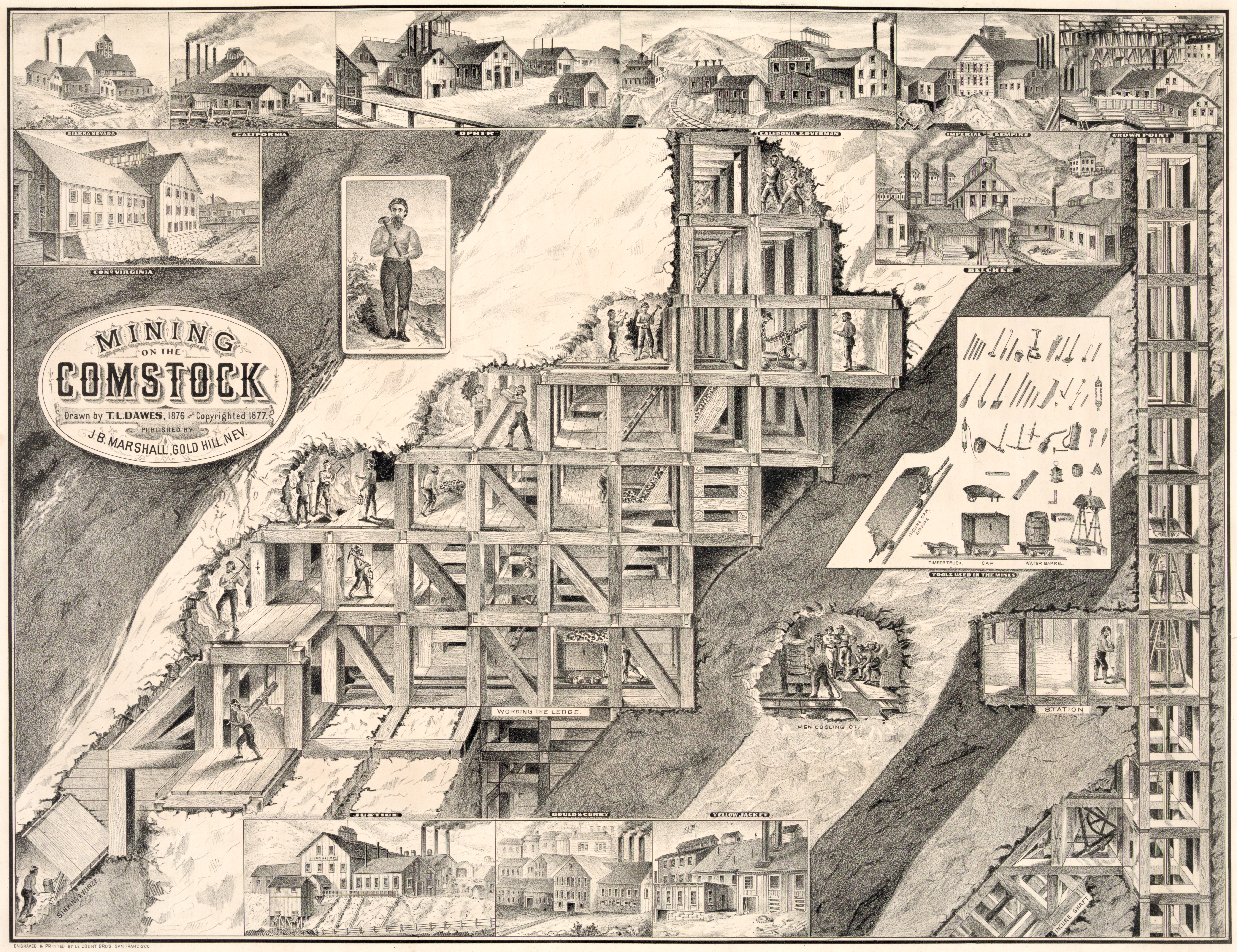
Comstock Lode
The Comstock Lode is a lode of silver ore located under the eastern slope of Mount Davidson, a peak in the Virginia Range in Virginia City, Nevada (then western Utah Territory), which was the first major discovery of silver ore in the United States and named after American miner Henry Comstock. After the discovery was made public in 1859, it sparked a silver rush of prospectors to the area, scrambling to stake their claims. The discovery caused considerable excitement in California and throughout the United States, the greatest since the California Gold Rush in 1849. Mining camps soon thrived in the vicinity, which became bustling commercial centers, including Virginia City and Gold Hill. The Comstock Lode is notable not just for the immense fortunes it generated and the large role those fortunes had in the growth of Nevada and San Francisco, but also for the advances in mining technology that it spurred, such as square set timbering and the Washoe process for ext ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mexico
Mexico, officially the United Mexican States, is a country in North America. It is the northernmost country in Latin America, and borders the United States to the north, and Guatemala and Belize to the southeast; while having maritime boundary, maritime boundaries with the Pacific Ocean to the west, the Caribbean Sea to the southeast, and the Gulf of Mexico to the east. Mexico covers 1,972,550 km2 (761,610 sq mi), and is the List of countries by area, thirteenth-largest country in the world by land area. With a population exceeding 130 million, Mexico is the List of countries by population, tenth-most populous country in the world and is home to the Hispanophone#Countries, largest number of native Spanish speakers. Mexico City is the capital and List of cities in Mexico, largest city, which ranks among the List of cities by population, most populous metropolitan areas in the world. Human presence in Mexico dates back to at least 8,000 BC. Mesoamerica, considered a cradle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gravity
Relative density, also called specific gravity, is a dimensionless quantity defined as the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for solids and liquids is nearly always measured with respect to water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (abbreviated r.d. or RD) is preferred in SI, whereas the term "specific gravity" is gradually being abandoned. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |