|
Antithyroid Drugs
An antithyroid agent is a hormone inhibitor acting upon thyroid hormones. The main antithyroid drugs are carbimazole (in the UK), methimazole (in the US), and propylthiouracil (PTU). A less common antithyroid agent is potassium perchlorate. Classification based on mechanisms of action The mechanisms of action of antithyroid drugs are not completely understood. Based on their mechanisms of action, the drugs are classified into following six classes. Thyroid hormone synthesis inhbitors These drugs probably inhibit the enzyme thyroid peroxidase ( thyroperoxidase), decreasing iodide oxidation, iodination of tyrosyl residues in thyroglobulin, and coupling of iodotyrosyl and iodothyronyl residues. It is thought that they inhibit the thyroperoxidase-catalyzed oxidation reactions by acting as substrates for the postulated peroxidase-iodine complex, thus competitively inhibiting the interaction with the amino acid tyrosine. The most common drugs in this class are thioamides, which incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hormone
A hormone (from the Ancient Greek, Greek participle , "setting in motion") is a class of cell signaling, signaling molecules in multicellular organisms that are sent to distant organs or tissues by complex biological processes to regulate physiology and behavior. Hormones are required for the normal development of animals, plants and fungi. Due to the broad definition of a hormone (as a signaling molecule that exerts its effects far from its site of production), numerous kinds of molecules can be classified as hormones. Among the substances that can be considered hormones, are eicosanoids (e.g. prostaglandins and thromboxanes), steroids (e.g. Estrogen, oestrogen and brassinosteroid), amino acid derivatives (e.g. epinephrine and auxin), protein or peptides (e.g. insulin and CLE peptides), and gases (e.g. ethylene and nitric oxide). Hormones are used to communicate between organ (anatomy), organs and Tissue (biology), tissues. In vertebrates, hormones are responsible for regulating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not. The l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioisotopes
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nucl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diabetes Insipidus
Diabetes insipidus (DI) is a condition characterized by large amounts of dilute urine and increased thirst. The amount of urine produced can be nearly 20 liters per day. Reduction of fluid has little effect on the concentration of the urine. Complications may include dehydration or seizures. There are four types of DI, each with a different set of causes. # Central DI (CDI), now known as arginine vasopressin deficiency (AVP-D), is due to a lack of vasopressin (antidiuretic hormone) production. This can be due to injury to the hypothalamus or pituitary gland or due to genetics. # Nephrogenic DI (NDI), also known as arginine vasopressin resistance (AVP-R), occurs when the kidneys do not respond properly to vasopressin. # Dipsogenic DI is a result of excessive fluid intake due to damage to the hypothalamic thirst mechanism. It occurs more often in those with certain psychiatric disorders or on certain medications. # Gestational DI occurs only during pregnancy. Diagnosis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
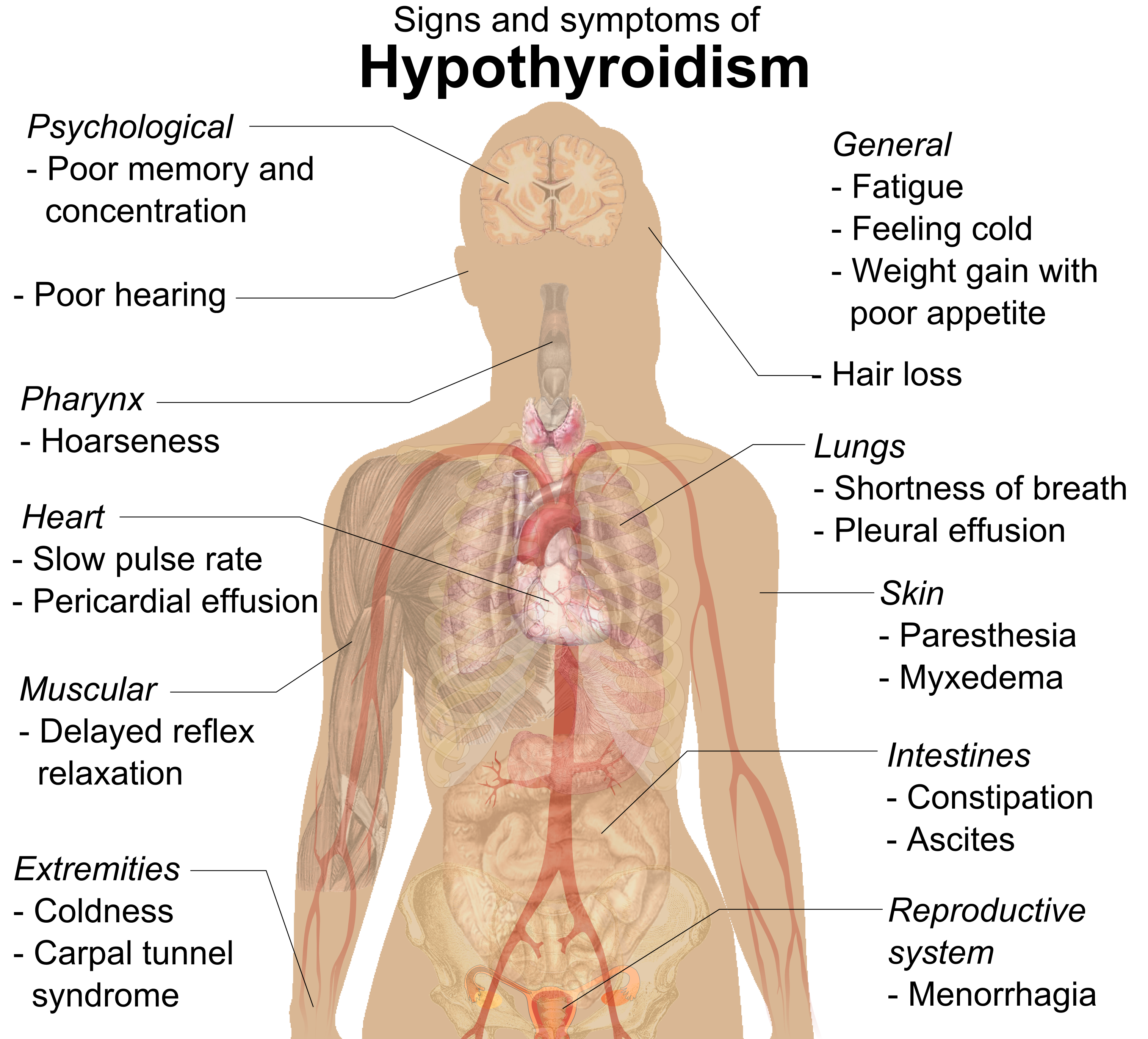
Hypothyroidism
Hypothyroidism is an endocrine disease in which the thyroid gland does not produce enough thyroid hormones. It can cause a number of symptoms, such as cold intolerance, poor ability to tolerate cold, fatigue, extreme fatigue, muscle aches, constipation, slow heart rate, Depression (mood), depression, and weight gain. Occasionally there may be swelling of the front part of the neck due to goiter. Untreated cases of hypothyroidism during pregnancy can lead to delays in child development, growth and intellectual development in the baby or congenital iodine deficiency syndrome. Worldwide, iodine deficiency, too little iodine in the diet is the most common cause of hypothyroidism. Hashimoto's thyroiditis, an autoimmune disease where the body's immune system reacts to the thyroid gland, is the most common cause of hypothyroidism in countries with sufficient dietary iodine. Less common causes include previous treatment with iodine-131, radioactive iodine, injury to the hypothalamus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Toxicity
Lithium toxicity, also known as lithium overdose, is the condition of having too much lithium. Symptoms may include a tremor, increased reflexes, trouble walking, kidney problems, and an altered level of consciousness. Some symptoms may last for a year after levels return to normal. Complications may include serotonin syndrome. Lithium toxicity can occur due to excessive intake or decreased excretion. Excessive intake may be either a suicide attempt or accidental. Decreased excretion may occur as a result of dehydration such as from vomiting or diarrhea, a low sodium diet, or from kidney problems. The diagnosis is generally based on symptoms and supported by a lithium level in blood serum of greater than 1.2 mEq/L. Gastric lavage and whole bowel irrigation may be useful if done early. Activated charcoal (medication), Activated charcoal is not effective. For severe toxicity hemodialysis is recommended. The risk of death is generally low. Acute toxicity generally has better outcom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium (medication)
Certain lithium compounds, also known as lithium salts, are used as psychiatric medication, primarily for bipolar disorder and for major depressive disorder. Lithium is taken orally (by mouth). Common side effects include increased urination, shakiness of the hands, and increased thirst. Serious side effects include hypothyroidism, diabetes insipidus, and lithium toxicity. Blood level monitoring is recommended to decrease the risk of potential toxicity. If levels become too high, diarrhea, vomiting, poor coordination, sleepiness, and ringing in the ears may occur. Lithium is teratogenic and can cause birth defects at high doses, especially during the first trimester of pregnancy. The use of lithium while breastfeeding is controversial; however, many international health authorities advise against it, and the long-term outcomes of perinatal lithium exposure have not been studied. The American Academy of Pediatrics lists lithium as contraindicated for pregnancy and lactat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
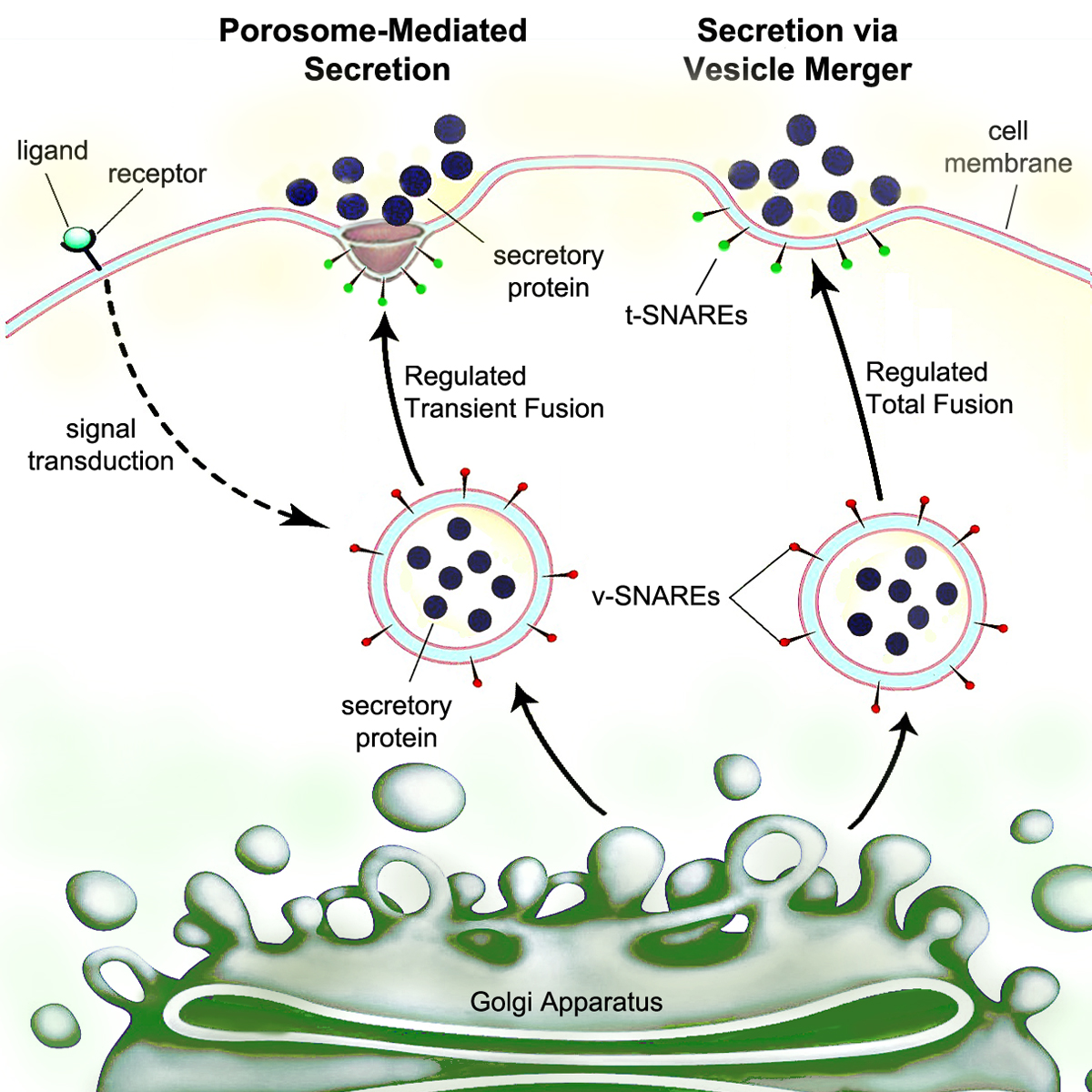
Secretion
Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures embedded in the cell membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell. Secretion in bacterial species means the transport or translocation of effector molecules. For example: proteins, enzymes or toxins (such as cholera toxin in pathogenic bacteria e.g. '' Vibrio cholerae'') from across the interior (cytoplasm or cytosol) of a bacterial cell to its exterior. Secretion is a very important mechanism in bacterial functioning and operation in their natural surrounding environment for adaptation and survival. In eukaryotic cells ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Chemical structure The nitrate anion is the conjugate acid, conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of Resonance (chemistry), resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by three resonance structures: Che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiocyanate
Thiocyanates are salts containing the thiocyanate anion (also known as rhodanide or rhodanate). is the conjugate base of thiocyanic acid. Common salts include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrotechnics. Thiocyanate is analogous to the cyanate ion, , wherein oxygen is replaced by sulfur. is one of the pseudohalides, due to the similarity of its reactions to that of halide ions. Thiocyanate used to be known as rhodanide (from a Greek word for rose) because of the red colour of its complexes with iron. Thiocyanate is produced by the reaction of elemental sulfur or thiosulfate with cyanide: : : The second reaction is catalyzed by thiosulfate sulfurtransferase, a hepatic mitochondrial enzyme, and by other sulfur transferases, which together are responsible for around 80% of cyanide metabolism in the body. Oxidation of thiocyanate inevitably produces hydrogen sulfate. The other product depe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pertechnetate
The pertechnetate ion () is an oxyanion with the chemical formula . It is often used as a convenient water-soluble source of isotopes of the radioactive element technetium (Tc). In particular it is used to carry the Technetium-99m, 99mTc isotope (half-life 6 hours) which is commonly used in nuclear medicine in several nuclear scanning procedures. Pertechnetate is poorly hydrated as [TcO4(H2O)n]− and [TcO4(H2O)n-m]−[H3O]+m (n = 1–50, m = 1–4) clusters that have been demonstrated by simulation with DFT. First hydration shell of TcO4− is asymmetric and contains no more than 7 water molecules. Only three of the four oxygen atoms of TcO4− form hydrogen bonds with water molecules. A technetate(VII) salt is a chemical compound, compound containing this ion. Pertechnetate compounds are salts of pertechnetic acid, technetic(VII) acid. Pertechnetate is analogous to permanganate but it has little oxidizing power. Pertechnetate has higher oxidation power than perrhenate. Understa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |