|
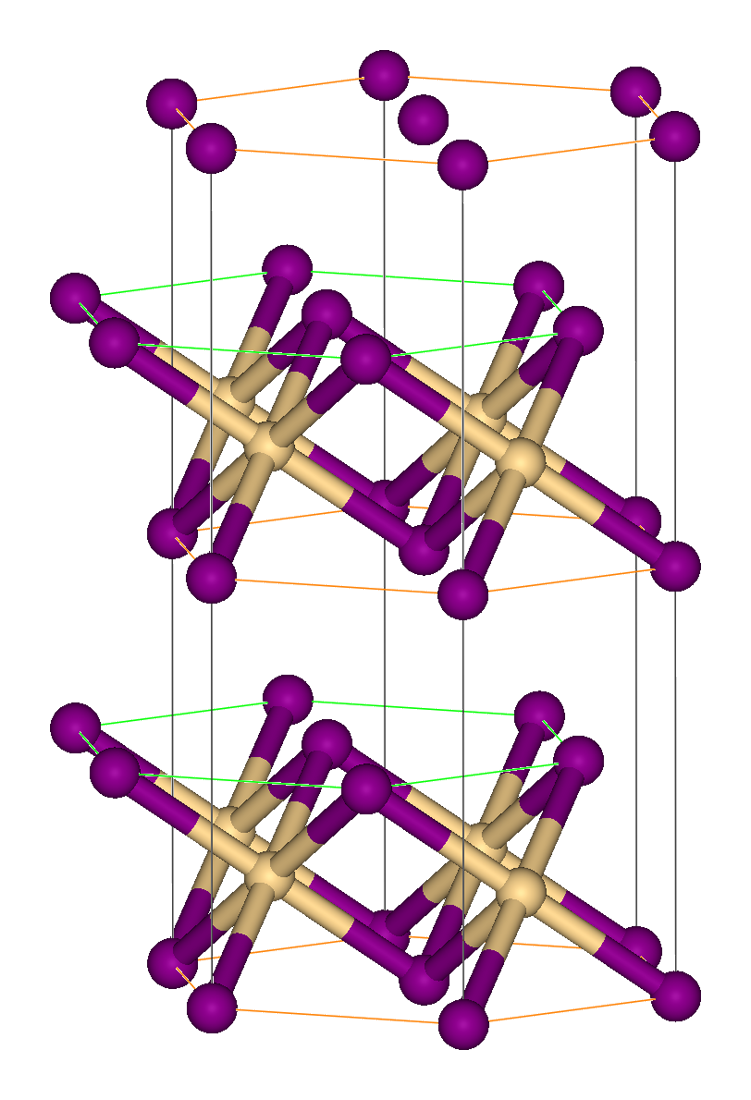
Anti-structure
In crystallography, an anti-structure is obtained from a salt structure by exchanging anion and cation positions. For instance, calcium fluoride, CaF2, crystallizes in a cubic motif called the fluorite structure. The same crystal structure is found in numerous ionic compounds with formula AB2, such as ceria (CeO2), zirconia (cubic ZrO2), uranium dioxide (UO2). In the corresponding ''anti-structure'', called the antifluorite structure, anions and cations are swapped, such as beryllium carbide (Be2C) or lithium oxide (Li2O), potassium sulfate Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, prov ... (K2SO4). Other anti-structures include: * anti- SnO2: Ti2N * anti- PbCl2: Co2P * anti- CdCl2: Co2N * anti- CdI2: Cs2O * anti- NbS2: Hf2S * anti- ReO3: Cu3N * anti- LaF3: Cu3P, Cu3As Refer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Fluoride
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white solid that is practically insoluble in water. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities. Chemical structure The compound crystallizes in a cubic motif called the fluorite structure. Ca2+ centres are eight-coordinate, being centred in a cube of eight F− centres. Each F− centre is coordinated to four Ca2+ centres in the shape of a tetrahedron. Although perfectly packed crystalline samples are colorless, the mineral is often deeply colored due to the presence of F-centers. The same crystal structure is found in numerous ionic compounds with formula AB2, such as CeO2, cubic ZrO2, UO2, ThO2, and PuO2. In the corresponding anti-structure, called the antifluorite structure, anions and cations are swapped, such as Be2C. Gas phase The gas phase is noteworthy for failing the predictions of VSEP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorite Structure
The fluorite structure refers to a common motif for compounds with the formula MX2. The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties o ... (FCC) structure. Many compounds, notably the common mineral fluorite (CaF2), adopt this structure. Many compounds with formula M2X have an antifluorite structure. In these the locations of the anions and cations are reversed relative to fluorite (an anti-structure); the anions occupy the FCC regular sites whereas the cations occupy the tetrahedral interstitial sites. For example, magnesium silicide, Mg2Si, has a lattice parameter of 6.338 Å with magnesium cations occupying the tetrahedral interstitial sites, in which each s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
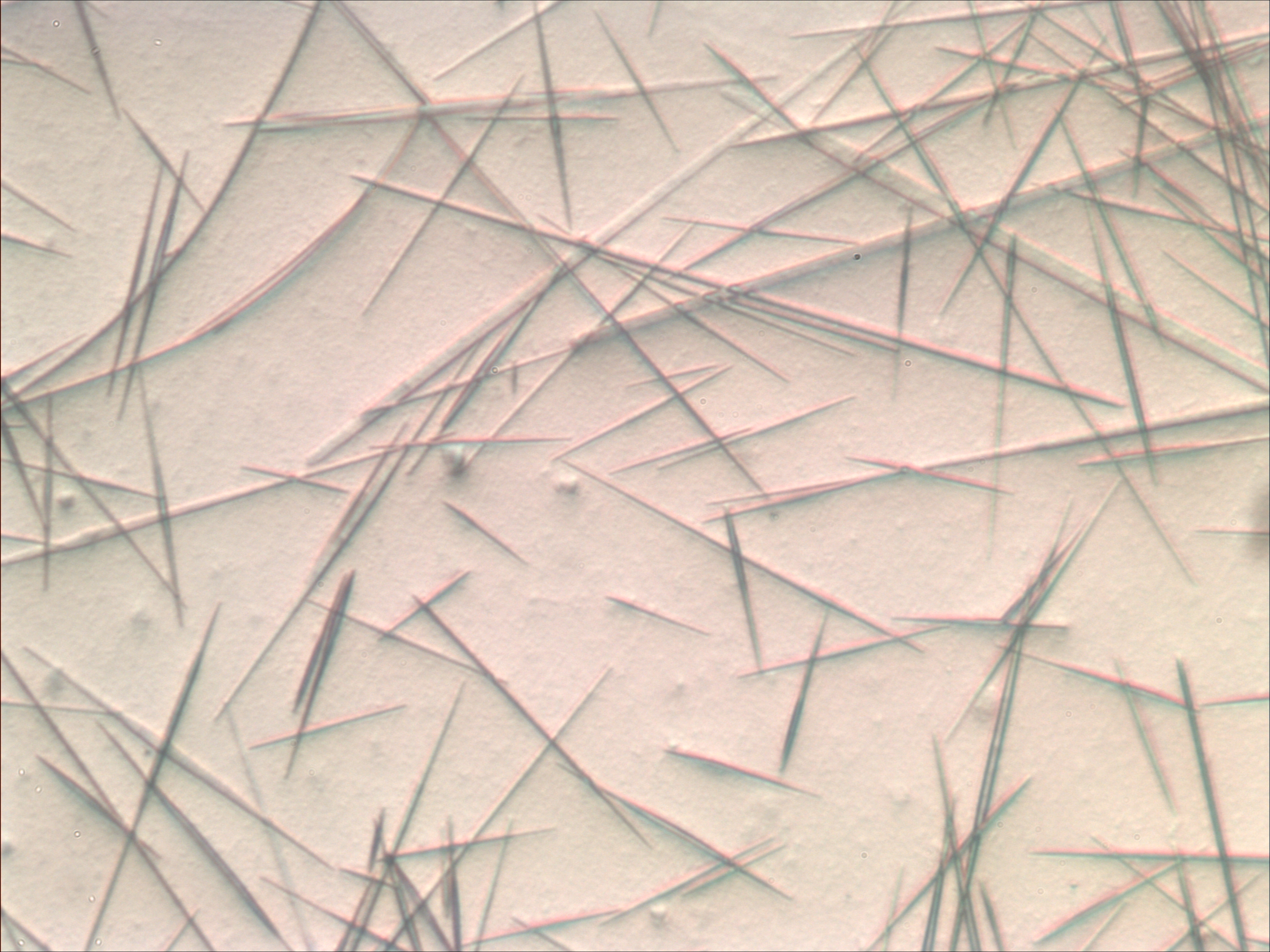
Lead(II) Chloride
Lead(II) chloride (PbCl2) is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It also occurs naturally in the form of the mineral cotunnite. Structure and properties In solid PbCl2, each lead ion is coordinated by nine chloride ions in a tricapped triangular prism formation — six lie at the vertices of a triangular prism and three lie beyond the centers of each rectangular prism face. The 9 chloride ions are not equidistant from the central lead atom, 7 lie at 280–309 pm and 2 at 370 pm. PbCl2 forms white orthorhombic needles. In the gas phase, PbCl2 molecules have a bent structure with the Cl–Pb–Cl angle being 98° and each Pb–-Cl bond distance being 2.44 Å. Such PbCl2 is emitted from internal combustion engines that use ethylene chloride-tetraethyllead additives for antiknock purposes. PbCl2 is sparingly soluble in water, solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum Fluoride
Lanthanum trifluoride is a refractory ionic compound of lanthanum and fluorine. The chemical formula is . The LaF3 structure Bonding is ionic with lanthanum highly coordinated. The cation sits at the center of a trigonal prism. Nine fluorine atoms are close: three at the bottom corners of the trigonal prism, three in the faces of the trigonal prism, and three at top corners of the trigonal prism. There are also two fluorides a little further away above and below the prism. The cation can be considered 9-coordinate or 11-coordinate. At 300 K, the structure allows the formation of Schottky defects with an activation energy of 0.07 eV, and free flow of fluoride ions with an activation energy of 0.45 eV, making the crystal unusually electrically conductive. The larger sized rare earth elements (lanthanides), which are those with smaller atomic number, also form trifluorides with the LaF3 structure. Some actinides do as well. Applications This white salt is somet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium Trioxide
Rhenium trioxide or rhenium(VI) oxide is an inorganic compound with the formula ReO3. It is a red solid with a metallic lustre that resembles copper in appearance. It is the only stable trioxide of the Group 7 elements ( Mn, Tc, Re). Preparation and structure Rhenium trioxide can be formed by reducing rhenium(VII) oxide with carbon monoxide at 200 °C or elemental rhenium at 400 °C. :Re2O7 + CO → 2 ReO3 + CO2 :3 Re2O7 + Re → 7 ReO3 Re2O7 can also be reduced with dioxane. Rhenium trioxide crystallizes with a primitive cubic unit cell, with a lattice parameter of 3.742 Å (374.2 pm). The structure of ReO3 is similar to that of perovskite (ABO3), without the large A cation at the centre of the unit cell. Each rhenium center is surrounded by an octahedron defined by six oxygen centers. These octahedra share corners to form the 3-dimensional structure. The coordination number of O is 2, because each oxygen atom has 2 neighbouring Re atoms., p. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niobium Disulfide
Niobium disulfide is the chemical compound with the formula NbS2. It is a black layered solid that can be exfoliated into ultrathin grayish sheets similar to other transition metal dichalcogenides. These layers exhibit superconductivity Superconductivity is a set of physical properties observed in superconductors: materials where Electrical resistance and conductance, electrical resistance vanishes and Magnetic field, magnetic fields are expelled from the material. Unlike an ord ..., where the transition temperature increases from ca. 2 to 6 K with the layer thickness increasing from 6 to 12 nm, and then saturates with thickness. References {{Sulfides Niobium(IV) compounds Disulfides Transition metal dichalcogenides Monolayers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Oxide
Caesium oxide (IUPAC name), or cesium oxide, describes inorganic compounds composed of caesium and oxygen. Several binary (containing only Cs and O) oxides of caesium are known.. Caesium oxide may refer to: * Caesium suboxides () * Caesium monoxide (, the most common oxide) * Caesium peroxide () * Caesium sesquioxide () * Caesium superoxide () * Caesium ozonide Caesium ozonide is an oxygen-rich chemical compound of caesium, with the chemical formula . It consists of caesium cations and ozonide anions . It can be formed by reacting ozone with caesium superoxide: : The compound reacts strongly with any ... () References {{Authority control Caesium compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Iodide
Cadmium iodide is an inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects. Preparation Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine. Applications Historically, cadmium iodide was used as a catalyst for the Henkel process, a high-temperature isomerisation of dipotassium phthalate to yield the terephthalate. The salt was then treated with acetic acid to yield potassium acetate and commercially valuable terephthalic acid. While uneconomical compared to the production of terephthalic acid from ''p''-xylene, the Henkel method has been proposed as a potential route to produce terephthalic acid from furfural. As existing B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Chloride
Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and the hemipentahydrate CdCl2•2.5H2O. Structure Anhydrous Anhydrous cadmium chloride forms a layered structure consisting of octahedral Cd2+ centers linked with chloride ligands. Cadmium iodide, CdI2, has a similar structure, but the iodide ions are arranged in a HCP lattice, whereas in CdCl2 the chloride ions are arranged in a CCP lattice.N. N. Greenwood, A. Earnshaw, ''Chemistry of the Elements'', 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997. Hydrates The anhydrous form absorbs moisture from the air to form various hydrates. Three of these hydrates have been examined by X-ray crystallography. Chemical properties Cadmium chloride di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Sulfate
Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, providing both potassium and sulfur. History Potassium sulfate (K2SO4) has been known since early in the 14th century. It was studied by Glauber, Boyle, and Tachenius. In the 17th century, it was named ''arcanuni'' or ''sal duplicatum'', as it was a combination of an acid salt with an alkaline salt. It was also known as ''vitriolic tartar'' and ''Glaser's salt'' or ''sal polychrestum Glaseri'' after the pharmaceutical chemist Christopher Glaser who prepared it and used medicinally. Known as ''arcanum duplicatum'' ("double secret") or ''panacea duplicata'' in pre-modern medicine, it was prepared from the residue ('' caput mortuum'') left over from the production of aqua fortis (nitric acid, HNO3) from nitre (potassium nitrate, KNO3) an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin(IV) Oxide
Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid. Structure Tin(IV) oxide crystallises with the rutile structure. As such the tin atoms are six coordinate and the oxygen atoms three coordinate. SnO2 is usually regarded as an oxygen-deficient n-type semiconductor. Hydrous forms of SnO2 have been described as stannic acid. Such materials appear to be hydrated particles of SnO2 where the composition reflects the particle size. Preparation Tin(IV) oxide occurs naturally. Synthetic tin(IV) oxide is produced by burning tin metal in air. Annual production is in the range of 10 kilotons. SnO2 is reduced industrially to the metal with carbon in a reverberatory furnace at 1200–1300 °C. Reactions The reaction from tin(IV) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |