|
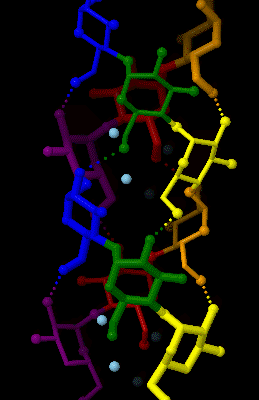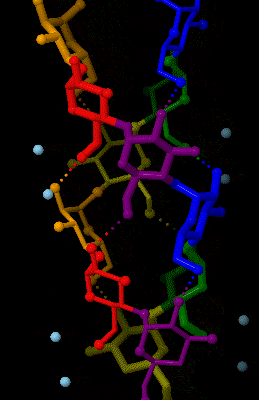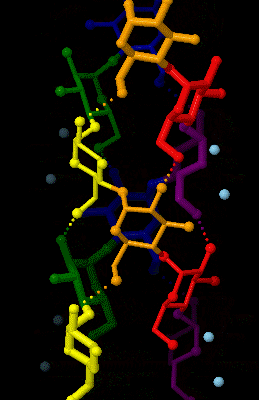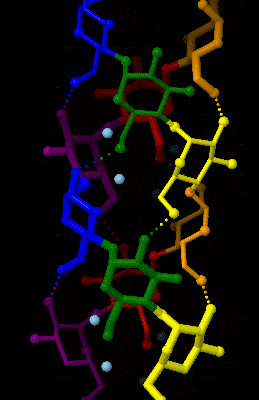
Amylose 3Dprojection
Amylose is a polysaccharide made of α-D-glucose units, bonded to each other through α(1→4) glycosidic bonds. It is one of the two components of starch, making up approximately 20–25% of it. Because of its tightly packed helical structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch. Structure Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The structural formula of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands. There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation, found both in starch granules and in hydrated amylose (when starch is coo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars (monosaccharides or oligosaccharides). They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as hemicellulose and chitin. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water. When all the monosaccharides in a polysaccharide are the same type, the polysaccharide is called a homopolysaccharide or homoglycan, but when more t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltotriose
Maltotriose is a trisaccharide (three-part sugar) consisting of three glucose molecules linked with α-1,4 glycosidic bonds. It is most commonly produced by the digestive enzyme alpha-amylase (a common enzyme in human saliva) on amylose in starch. The creation of both maltotriose and maltose during this process is due to the random manner in which alpha amylase hydrolyses α-1,4 glycosidic bonds. It is the shortest chain oligosaccharide that can be classified as maltodextrin Maltodextrin is a name shared by two different families of chemicals. Both families are glucose polymers (also called ''dextrose polymers'' or ''Dextrin, dextrins''), but have little chemical or nutritional similarity. The digestible maltodextr .... References Trisaccharides Types of sugar {{Carbohydrate-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine Test
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek , meaning 'violet'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compounds, it has also found favour as a non-toxic radiocontrast material. Because of the specifici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wavelengths
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats. In other words, it is the distance between consecutive corresponding points of the same '' phase'' on the wave, such as two adjacent crests, troughs, or zero crossings. Wavelength is a characteristic of both traveling waves and standing waves, as well as other spatial wave patterns. The inverse of the wavelength is called the ''spatial frequency''. Wavelength is commonly designated by the Greek letter lambda (''λ''). For a modulated wave, ''wavelength'' may refer to the carrier wavelength of the signal. The term ''wavelength'' may also apply to the repeating envelope of modulated waves or waves formed by interference of several sinusoids. Assuming a sinusoidal wave moving at a fixed wave speed, wavelength is inversely proportional to the frequency of the wave: waves with higher frequencies have shorter wavelengths, and lower frequenci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek , meaning 'violet'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compounds, it has also found favour as a non-toxic radiocontrast material. Because of the spec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
White Sauce
White is the lightest color and is achromatic (having no chroma). It is the color of objects such as snow, chalk, and milk, and is the opposite of black. White objects fully (or almost fully) reflect and scatter all the visible wavelengths of light. White on television and computer screens is created by a mixture of red, blue, and green light. The color white can be given with white pigments, especially titanium dioxide. In ancient Egypt and ancient Rome, priestesses wore white as a symbol of purity, and Romans wore white togas as symbols of citizenship. In the Middle Ages and Renaissance a white unicorn symbolized chastity, and a white lamb sacrifice and purity. It was the royal color of the kings of France as well as the flag of monarchist France from 1815 to 1830, and of the monarchist movement that opposed the Bolsheviks during the Russian Civil War (1917–1922). Greek temples and Roman temples were faced with white marble, and beginning in the 18th century, wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthan Gum
Xanthan gum () is a polysaccharide with many industrial uses, including as a common food additive. It is an effective thickening agent and stabilizer that prevents ingredients from separating. It can be produced from simple sugars by fermentation and derives its name from the species of bacteria used, '' Xanthomonas campestris''. History Xanthan gum was discovered by Allene Rosalind Jeanes and her research team at the United States Department of Agriculture and brought into commercial production by CP Kelco in the early 1960s under the trade name Kelzan, remaining the only manufacturer in the US. It was approved for use in foods in 1968 and is accepted as a safe food additive in the US, Canada, European countries, and many other countries, with E number E415 and CAS number 11138-66-2. Xanthan gum derives its name from the species of bacteria used during the fermentation process, '' Xanthomonas campestris''. Uses The addition of 1% xanthan gum can produce a significant incr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alginate
Alginic acid, also called algin, is a naturally occurring, edible polysaccharide found in brown algae. It is hydrophilic and forms a viscous gum when hydrated. When the alginic acid binds with sodium and calcium ions, the resulting salts are known as alginates. Its colour ranges from white to yellowish-brown. It is sold in filamentous, granular, or powdered forms. It is a significant component of the biofilms produced by the bacterium '' Pseudomonas aeruginosa'', a major pathogen found in the lungs of some people who have cystic fibrosis. The biofilm and ''P. aeruginosa'' have a high resistance to antibiotics, but are susceptible to inhibition by macrophages. Alginate was discovered by British chemical scientist E. C. C. Stanford in 1881, and he patented an extraction process for it in the same year. The alginate was extracted, in the original patent, by first soaking the algae in water or diluted acid, then extracting the alginate by soaking it in sodium carbonate, and fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carrageenan
Carrageenans or carrageenins ( ; ) are a family of natural linear sulfation, sulfated polysaccharides. They are extracted from red algae, red edible seaweeds. Carrageenans are widely used in the food industry, for their gelling, thickening, and stabilizing properties. Their main application is in dairy and meat products, due to their strong binding to food proteins. Carrageenans have emerged as a promising candidate in tissue engineering and regenerative medicine applications as they resemble animal glycosaminoglycans (GAGs). They are used for tissue engineering, wound coverage, and drug delivery. Carrageenans contain 15–40% ester-sulfate content, which makes them anionic polysaccharides. They can be mainly categorized into three classes based on their sulfate content. Kappa-carrageenan has one sulfate group per disaccharide, iota-carrageenan has two, and lambda-carrageenan has three. A common seaweed used for manufacturing the hydrophilic colloids to produce carrageenan is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for example, syrup has a higher viscosity than water. Viscosity is defined scientifically as a force multiplied by a time divided by an area. Thus its SI units are newton-seconds per metre squared, or pascal-seconds. Viscosity quantifies the internal friction, frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's center line than near its walls. Experiments show that some stress (physics), stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Syneresis (chemistry)
Syneresis (also spelled 'synæresis' or 'synaeresis'), in chemistry, is the solvent extraction, extraction or expulsion of a liquid from a gel, such as when Serum (blood), serum drains from a contracting clot of blood. Another example of syneresis is the collection of whey on the surface of yogurt. Syneresis can also be observed when the amount of diluent in a swollen polymer exceeds the solubility limit as the temperature changes. A household example of this is the counterintuitive expulsion of water from dry gelatin when the temperature increases. Syneresis has also been proposed as the mechanism of formation for the Silicon dioxide, amorphous silica composing the frustule of diatoms. Examples In the processing of dairy milk, for example during cheese making, syneresis is the formation of the curd due to the sudden removal of the hydrophilic peptides, macropeptides, which causes an imbalance in intermolecular forces. Bonds between hydrophobic sites start to develop and are enfor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Compounds
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's rule. Aromatic compounds have the following general properties: * Typically unreactive * Often non polar and hydrophobic * High carbon-hydrogen ratio * Burn with a strong sooty yellow flame, due to high C:H ratio * Undergo electrophilic substitution reactions and nucleophilic aromatic substitutions Arenes are typically split into two categories - benzoids, that contain a benzene derivative and follow the benzene ring model, and non-benzoids that contain other aromatic cyclic derivatives. Aromatic compounds are commonly used in organic synthesis and are involved in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |