|
Amyloid Beta
Amyloid beta (Aβ, Abeta or beta-amyloid) denotes peptides of 36–43 amino acids that are the main component of the amyloid plaques found in the brains of people with Alzheimer's disease. The peptides derive from the amyloid-beta precursor protein (APP), which is cleaved by Beta-secretase 1, beta secretase and gamma secretase to yield Aβ in a cholesterol-dependent process and substrate presentation. Both neurons and oligodendrocytes produce and release Aβ in the brain, contributing to formation of amyloid plaques. Aβ molecules can aggregate to form flexible soluble oligomers which may exist in several forms. It is now believed that certain misfolded oligomers (known as "seeds") can induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a prion infection. The oligomers are toxic to nerve cells. The other protein implicated in Alzheimer's disease, tau protein, also forms such prion-like misfolded oligomers, and there is some ev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloid Precursor Protein
Amyloid-beta precursor protein (APP) is an integral membrane protein expressed in many biological tissue, tissues and concentrated in the synapses of neurons. It functions as a cell surface receptor and has been implicated as a regulator of synapse formation, neural plasticity, antimicrobial activity, and iron export. It is coded for by the gene ''APP'' and regulated by substrate presentation. APP is best known as the precursor molecule whose proteolysis generates amyloid beta (Aβ), a polypeptide containing 37 to 49 amino acid residues, whose Amyloid#Structure, amyloid fibrillar form is the primary component of amyloid plaques found in the brains of Alzheimer's disease patients. Genetics Amyloid-beta precursor protein is an ancient and highly Conserved sequence, conserved protein. In humans, the gene ''APP'' is located on chromosome 21 and contains 18 exons spanning 290 kilobases. Several alternative splicing isoforms of APP have been observed in humans, ranging in length ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils. Cholesterol is biosynthesis, biosynthesized by all animal Cell (biology)#Eukaryotic cells, cells and is an essential structural and cholesterol signaling, signaling component of animal cell membranes. In vertebrates, hepatocyte, hepatic cells typically produce the greatest amounts. In the brain, astrocytes produce cholesterol and transport it to neurons. It is absent among prokaryotes (bacteria and archaea), although there are some exceptions, such as ''Mycoplasma'', which require cholesterol for growth. Cholesterol also serves as a Precursor (chemistry), precursor for the biosynthesis of steroid hormones, bile acid and vitamin D. Elevated levels of cholesterol in the blood, especially when bound to low-density lipoprotein (LDL, often referred to as "bad cholesterol"), may increase the risk of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parenchymal
upright=1.6, Lung parenchyma showing damage due to large subpleural bullae. Parenchyma () is the bulk of functional substance in an animal organ such as the brain or lungs, or a structure such as a tumour. In zoology, it is the tissue that fills the interior of flatworms. In botany, it is some layers in the cross-section of the leaf. Etymology The term ''parenchyma'' is Neo-Latin from the Ancient Greek word meaning 'visceral flesh', and from meaning 'to pour in' from 'beside' + 'in' + 'to pour'. Originally, Erasistratus and other anatomists used it for certain human tissues. Later, it was also applied to plant tissues by Nehemiah Grew. Structure The parenchyma is the ''functional'' parts of an organ, or of a structure such as a tumour in the body. This is in contrast to the stroma, which refers to the ''structural'' tissue of organs or of structures, namely, the connective tissues. Brain The brain parenchyma refers to the functional tissue in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
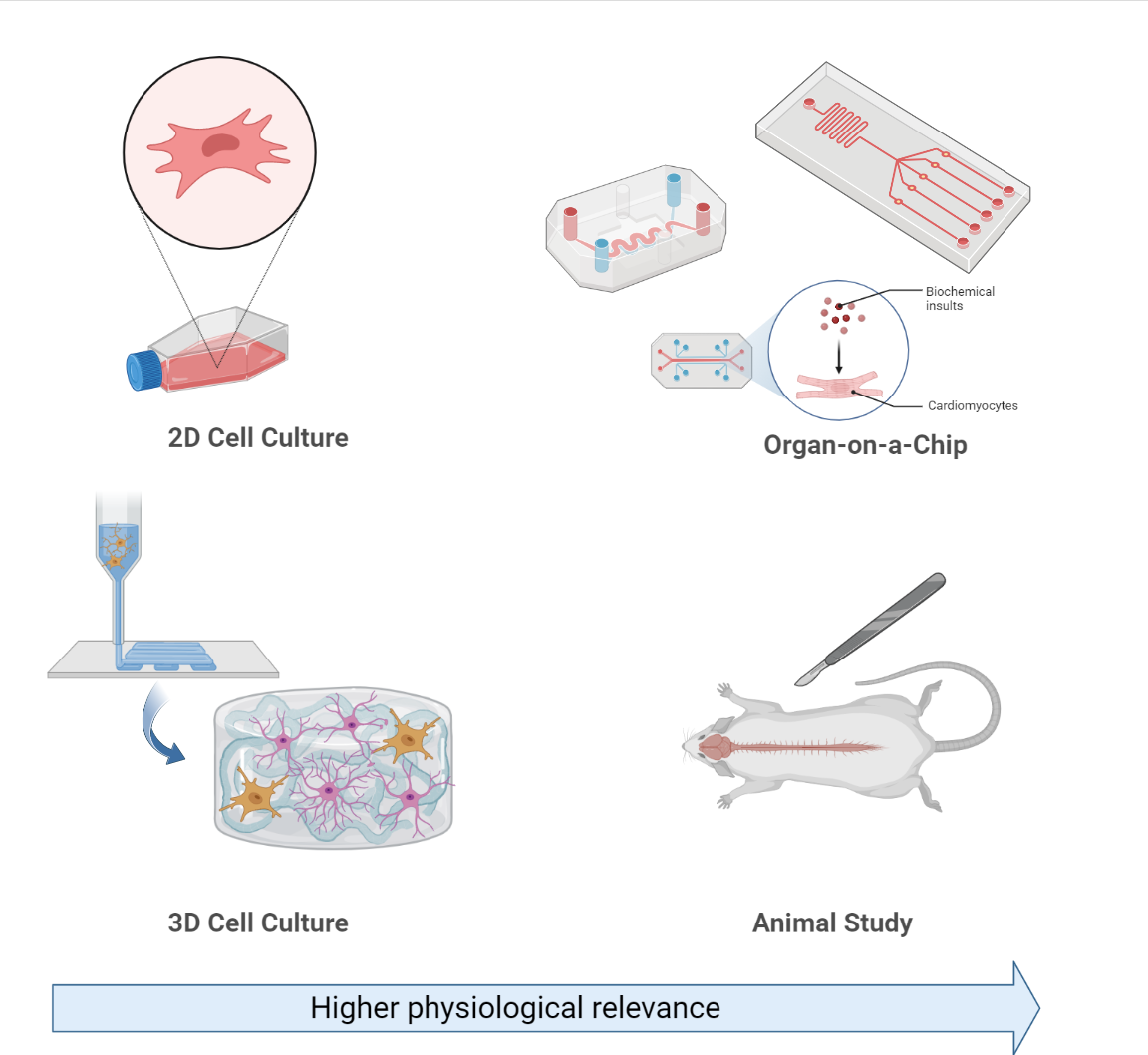
Experimental Models Of Alzheimer's Disease
Experimental models of Alzheimer's disease are organism or cellular models used in research to investigate biological questions about Alzheimer's disease as well as develop and test novel therapeutic treatments. Alzheimer's disease is a progressive neurodegenerative disorder associated with aging, which occurs both sporadically (the most common form of diagnosis) or due to familial passed mutations in genes associated with Alzheimer's pathology. Common symptoms associated with Alzheimer's disease include: memory loss, confusion, and mood changes. As Alzheimer's disease affects around 55 million patients globally and accounts for approximately 60-70% of all dementia cases, billions of dollars are spent yearly towards research to better understand the biological mechanisms of the disease as well as develop effective therapeutic treatments for it. Researchers commonly use post-mortem human tissue or experimental models to conduct experiments relating to Alzheimer's disease. Experimenta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteinopathy
In medicine, proteinopathy ( 'pref''. protein -pathy 'suff''. disease proteinopathies ''pl''.; proteinopathic ''adj''), or proteopathy, protein conformational disorder, or protein misfolding disease, is a class of diseases in which certain proteins become structurally abnormal, and thereby disrupt the function of cells, tissues and organs of the body. Often the proteins fail to fold into their normal configuration; in this misfolded state, the proteins can become toxic in some way (a toxic gain-of-function) or they can lose their normal function. The proteinopathies include such diseases as Creutzfeldt–Jakob disease (and a variant associated with mad cow disease) and other prion diseases, Alzheimer's disease, Parkinson's disease, amyloidosis, multiple system atrophy, and a wide range of other disorders. The term ''proteopathy'' was first proposed in 2000 by Lary Walker and Harry LeVine. The concept of proteopathy can trace its origins to the mid-19th century, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Folding
Protein folding is the physical process by which a protein, after Protein biosynthesis, synthesis by a ribosome as a linear chain of Amino acid, amino acids, changes from an unstable random coil into a more ordered protein tertiary structure, three-dimensional structure. This structure permits the protein to become biologically functional or active. The folding of many proteins begins even during the translation of the polypeptide chain. The amino acids interact with each other to produce a well-defined three-dimensional structure, known as the protein's native state. This structure is determined by the amino-acid sequence or primary structure. The correct three-dimensional structure is essential to function, although some parts of functional proteins Intrinsically unstructured proteins, may remain unfolded, indicating that protein dynamics are important. Failure to fold into a native structure generally produces inactive proteins, but in some instances, misfolded proteins have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloid
Amyloids are aggregates of proteins characterised by a fibrillar morphology of typically 7–13 nm in diameter, a β-sheet secondary structure (known as cross-β) and ability to be stained by particular dyes, such as Congo red. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal structure and physiological functions ( misfolding) and form fibrous deposits within and around cells. These protein misfolding and deposition processes disrupt the healthy function of tissues and organs. Such amyloids have been associated with (but not necessarily as the cause of) more than 50 human diseases, known as amyloidosis, and may play a role in some neurodegenerative diseases. Some of these diseases are mainly sporadic and only a few cases are familial. Others are only familial. Some result from medical treatment. Prions are an infectious form of amyloids that can act as a templa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
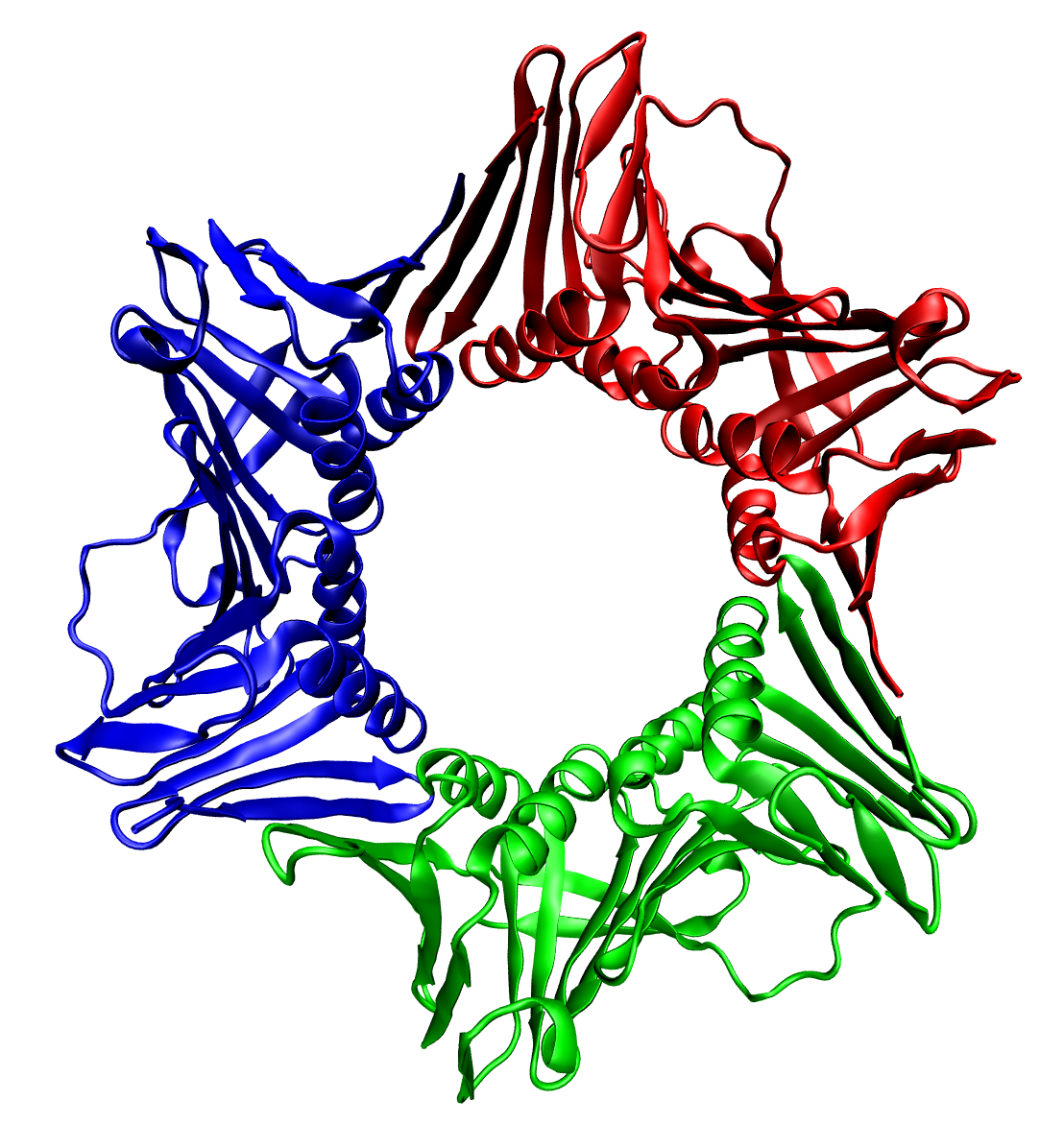
Protein Oligomer
Protein quaternary structure is the fourth (and highest) classification level of protein structure. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains (also referred to as subunits). Protein quaternary structure describes the number and arrangement of multiple folded protein subunits in a multi-subunit complex. It includes organizations from simple dimers to large homooligomers and complexes with defined or variable numbers of subunits. In contrast to the first three levels of protein structure, not all proteins will have a quaternary structure since some proteins function as single units. Protein quaternary structure can also refer to biomolecular complexes of proteins with nucleic acids and other cofactors. Description and examples Many proteins are actually assemblies of multiple polypeptide chains. The quaternary structure refers to the number and arrangement of the protein subunits ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerebral Amyloid Angiopathy
Cerebral amyloid angiopathy (CAA) is a form of angiopathy in which amyloid beta peptide deposits in the walls of small to medium blood vessels of the central nervous system and meninges. The term ''congophilic'' is sometimes used because the presence of the abnormal aggregations of amyloid can be demonstrated by microscopic examination of brain Biological tissue, tissue after staining with Congo red. The amyloid material is only found in the brain and as such the disease is not related to other forms of amyloidosis. Types Several familial variants exist. The condition is usually associated with amyloid beta. However, there are types involving other amyloid peptides: * The "Hereditary cystatin C amyloid angiopathy, Icelandic type" is associated with cystatin C amyloid (ACys). * The "British type" and "Danish type" are associated with British amyloid (ABri) and Danish amyloid (ADan) respectively. Both peptides are linked to mutations in ITM2B. * Familial amyloidosis-Finnish type is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerebral Blood Vessel
Cerebral circulation is the movement of blood through a network of cerebral arteries and veins supplying the brain. The rate of cerebral blood flow in an adult human is typically 750 milliliters per minute, or about 15% of cardiac output. Arteries deliver oxygenated blood, glucose and other nutrients to the brain. Veins carry "used or spent" blood back to the heart, to remove carbon dioxide, lactic acid, and other metabolic products. The neurovascular unit regulates cerebral blood flow so that activated neurons can be supplied with energy in the right amount and at the right time. Because the brain would quickly suffer damage from any stoppage in blood supply, the cerebral circulatory system has safeguards including autoregulation of the blood vessels. The failure of these safeguards may result in a stroke. The volume of blood in circulation is called the cerebral blood flow. Sudden intense accelerations change the gravitational forces perceived by bodies and can severely ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brain
The brain is an organ (biology), organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It consists of nervous tissue and is typically located in the head (cephalization), usually near organs for special senses such as visual perception, vision, hearing, and olfaction. Being the most specialized organ, it is responsible for receiving information from the sensory nervous system, processing that information (thought, cognition, and intelligence) and the coordination of motor control (muscle activity and endocrine system). While invertebrate brains arise from paired segmental ganglia (each of which is only responsible for the respective segmentation (biology), body segment) of the ventral nerve cord, vertebrate brains develop axially from the midline dorsal nerve cord as a brain vesicle, vesicular enlargement at the rostral (anatomical term), rostral end of the neural tube, with centralized control over all body segments. All vertebr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloid Plaque
Amyloid plaques (also known as neuritic plaques, amyloid beta plaques or senile plaques) are extracellular deposits of amyloid beta (Aβ) protein that present mainly in the grey matter of the brain. Degenerative neuronal elements and an abundance of microglia and astrocytes can be associated with amyloid plaques. Some plaques occur in the brain as a result of aging, but large numbers of plaques and neurofibrillary tangles are characteristic features of Alzheimer's disease. The plaques are highly variable in shape and size; in tissue sections immunostained for Aβ, they comprise a log-normal size distribution curve, with an average plaque area of 400–450 square micrometers (μm2). The smallest plaques (less than 200 μm2), which often consist of diffuse deposits of Aβ, are particularly numerous. Plaques form when Aβ misfolds and aggregates into oligomers and longer polymers, the latter of which are characteristic of amyloid. History In 1892, Paul Blocq and Gheor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |