|
Aldaric Acid
Aldaric acids are a group of sugar acids, where the terminal hydroxyl and carbonyl groups of the sugars have been replaced by terminal carboxylic acids, and are characterised by the formula HO2C-(CHOH)n-CO2H. Oxidation of just the aldehyde yields an aldonic acid while oxidation of just the terminal hydroxyl group yields an uronic acid.) Aldaric acids cannot form cyclic hemiacetals like unoxidized sugars, but they can sometimes form lactones. Synthesis and reactions Aldaric acids are usually synthesized by the oxidation of aldoses with nitric acid. In this reaction it is the open-chain (polyhydroxyaldehyde) form of the sugar that reacts. These compounds are of interest as bio-derived chemicals including to bio-derived polyesters. Structure Nomenclature of the aldaric acids is based on the sugars from which they are derived; for example, glucose is oxidized to glucaric acid and xylose to xylaric acid. Unlike their parent sugars, aldaric acids have the same functional group at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugar Acid
In organic chemistry, a sugar acid or acidic sugar is a monosaccharide with a carboxyl group at one end or both ends of its Polymer backbone, chain. Main classes of sugar acids include: * Aldonic acids, in which the aldehyde group () located at the initial end (Monosaccharide#Linear-chain monosaccharides, position 1) of an aldose is oxidized. * Ulosonic acids, in which the hydroxymethyl group () at the initial end of a 2-ketose is oxidized creating an α-ketoacid. * Uronic acids, in which the group at the terminal end of an aldose or ketose is oxidized. * Aldaric acids, in which both ends ( and ) of an aldose are oxidized. Examples Examples of sugar acids include: * Aldonic acids ** Glyceric acid (3C) ** Xylonic acid (5C) ** Gluconic acid (6C) ** Ascorbic acid (6C, unsaturated lactone) * Ulosonic acids ** Neuraminic acid (5-amino-3,5-dideoxy-D-glyceraldehyde, ''glycero''-D-galactose, ''galacto''-non-2-ulosonic acid) ** Ketodeoxyoctulosonic acid (KDO or 3-deoxy-D-mannose, ''mann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xylose
Xylose ( , , "wood") is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group. It is derived from hemicellulose, one of the main constituents of biomass. Like most sugars, it can adopt several structures depending on conditions. With its free aldehyde group, it is a reducing sugar. Structure The acyclic form of xylose has chemical formula . The cyclic hemiacetal isomers are more prevalent in solution and are of two types: the pyranoses, which feature six-membered rings, and the furanoses, which feature five-membered rings (with a pendant group). Each of these rings is subject to further isomerism, depending on the relative orientation of the anomeric hydroxy group. The dextrorotary form, -xylose, is the one that usually occurs endogenously in living things. A levorotary form, -xylose, can be synthesized. Occurren ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
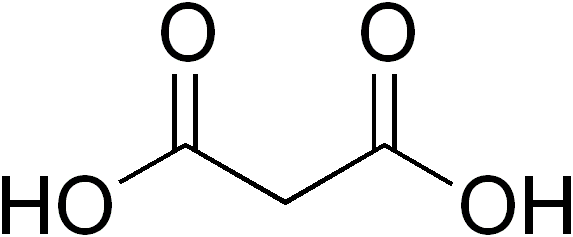
Dicarboxylic Acid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic.Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids. Dicarboxylic acids are usually colorless solids. A wide variety of dicarboxylic acids are used in industry. Adipic acid, for example, is a precursor to certain kinds of nylon. A wide variety of dicarboxylic acids are found in nature. Aspartic acid and glutamic acid are two amino acids found in all life. Succinic and fumaric acids are essential for metabolism. A large inventory of derivatives are known including many mono- and diesters, amides, etc. Partial list of saturated dicarboxylic acids Some common or illustrative examples : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adipic Acid
Adipic acid or hexanedioic acid is the organic compound with the formula C6H10O4. It a white crystalline powder at standard temperature and pressure. From an industrial perspective, it is the most important dicarboxylic acid at about 2.5 billion kilograms produced annually, mainly as a precursor for the production of nylon. Adipic acid otherwise rarely occurs in nature, but it is known as manufactured E number food additive E355. Salts and esters of adipic acid are known as adipates. Preparation and reactivity Adipic acid is produced by oxidation of a mixture of cyclohexanone and cyclohexanol, which is called KA oil, an abbreviation of ketone-alcohol oil. Nitric acid is the oxidant. The pathway is multistep. Early in the reaction, the cyclohexanol is converted to the ketone, releasing nitrous acid: : The cyclohexanone is then nitrosated, setting the stage for the scission of the C-C bond: : : Side products of the method include glutaric and succinic acids. Nitrous oxide is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactose
Galactose (, ''wikt:galacto-, galacto-'' + ''wikt:-ose#Suffix 2, -ose'', ), sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweetness, sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epimer of glucose. A galactose molecule linked with a glucose molecule forms a lactose molecule. Galactan is a polymeric form of galactose found in hemicellulose, and forming the core of the galactans, a class of natural polymeric carbohydrates. D-Galactose is also known as brain sugar since it is a component of glycoproteins (oligosaccharide-protein compounds) found in Nerve tissue, nerve tissue. Etymology The word ''galactose'' was coined by Charles Weissman in the mid-19th century and is derived from Greek language, Greek , , and the generic chemical suffix for sugars ''-ose''. The etymology is comparable to that of the word ''lactose'' in that both contain roots meaning "milk sugar". Lactose is a disaccharide of galactose plus glucose. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities which are mirror images of each other and non-superposable. Enantiomer molecules are like right and left hands: one cannot be superposed onto the other without first being converted to its mirror image. It is solely a relationship of chirality (chemistry), chirality and the permanent three-dimensional relationships among molecules or other chemical structures: no amount of re-orientation of a molecule as a whole or conformational isomerism, conformational change converts one chemical into its enantiomer. Chemical structures with chirality rotate plane-polarized light. A mixture of equal amounts of each enantiomer, a ''racemic mixture'' or a ''racemate'', does not rotate light. Stereoisomers include both enantiomers and diastereomers. Diaste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotation (geometry), rotations, translation (geometry), translations, and some Conformational isomerism, conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physics, physical properties, except that they often have opposite optical activity, optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic mixture, racem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical Activity
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circular birefringence and circular dichroism are the manifestations of optical activity. Optical activity occurs only in chiral materials, those lacking microscopic mirror symmetry. Unlike other sources of birefringence which alter a beam's state of polarization, optical activity can be observed in fluids. This can include gases or solutions of chiral molecules such as sugars, molecules with helical secondary structure such as some proteins, and also chiral liquid crystals. It can also be observed in chiral solids such as certain crystals with a rotation between adjacent crystal planes (such as quartz) or metamaterials. When looking at the source of light, the rotation of the plane of polarization may be either to the right (dextrorotatory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meso Form
A meso compound or meso isomer is an optically inactive isomer in a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters, the molecule is not chiral. A meso compound is superposable on its mirror image (not to be confused with superimposable, as any two objects can be superimposed over one another regardless of whether they are the same). Two objects can be superposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter. The name is derived from the Greek ''mésos'' meaning “middle”. For example, tartaric acid can exist as any of three stereoisomers depicted below in a Fischer projection. Of the four colored pictures at the top of the diagram, the first two represent the meso compound (the 2'' R'',3'' S'' and 2'' S'',3'' R'' isomers are equivalent), followed by the optically active pair of levotartaric acid (L-(''R,R'')-(+)-tartaric aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes such as formaldehyde and acetaldehyde are solubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fischer Projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry. The use of Fischer projections in non-carbohydrates is discouraged, as such drawings are ambiguous and easily confused with other types of drawing. The main purpose of Fischer projections is to show the chirality of a molecule and to distinguish between a pair of enantiomers. Some notable uses include drawing sugars and depicting isomers. Conventions All bonds are depicted as horizontal or vertical lines. The carbon chain is depicted vertically, with carbon atoms sometimes not shown and represented by the center of crossing lines (see figure below). The orientation of the carbon chain is so that the first carbon (C1) is at the top. In an aldose, C1 is the carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |