|
Acyl Cyanide
In organic chemistry, an acyl cyanide is a functional group with the Chemical formula, formula and Chemical structure, structure . It consists of an acyl group () attached to cyanide (). Examples include acetyl cyanide, formyl cyanide, and oxalyl dicyanide. Acyl cyanides are reagents in organic synthesis. Synthesis Classically acyl cyanides are produced by the salt metathesis reaction of acyl chlorides with sodium cyanide: :\ce + \ce \longrightarrow + \ce Alternatively, they can be produced by Dehydration reaction, dehydration of acyl aldoximes: :\ce\ce \longrightarrow + \ce Acetyl cyanide is also prepared by hydrocyanation of ketene: :\ce + \ce \longrightarrow \ce Reactions They are mild Acylation, acylating agents. With aqueous base, acyl cyanides break down to cyanide and the carboxylate: : + \ce \longrightarrow \ce + \ce + \ce With azides, acyl cyanides undergo the click reaction to give acyl tetrazoles.{{cite journal , doi=10.1002/1521-3773(20020617)41: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Cyanide
Sodium cyanide is a compound with the formula Na C N and the structure . It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. It is a moderately strong base. Production and chemical properties Sodium cyanide is produced by treating hydrogen cyanide with sodium hydroxide: : Worldwide production was estimated at 500,000 tons in the year 2006. Formerly it was prepared by the Castner process involving the reaction of sodium amide with carbon at elevated temperatures. : The structure of solid NaCN is related to that of sodium chloride. The anions and cations are each six-coordinate. Potassium cyanide (KCN) adopts a similar structure. When treated with acid, it forms the toxic gas hydrogen cyanide: : Because the salt is derived from a weak acid, sodium cyanide readily reverts to HCN by hydrolysis; the moist solid emits smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Groups
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrazole
A tetrazole is a chemical synthesis, synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound - a whitish crystalline powder with the formula CH2N4, of which three isomers exist. Structure and bonding Three isomers of the parent tetrazole exist, differing in the position of the double bonds: 1''H''-, 2''H''-, and 5''H''-tetrazole. The 1''H''- and 2''H''- isomers are tautomers, with the equilibrium lying on the side of 1''H''-tetrazole in the solid phase. In the gas phase, 2''H''-tetrazole dominates. These isomers can be regarded as aromatic, with 6 π-electrons, while the 5''H''-isomer is nonaromatic. Phosphorus analogs do not have the same electronic nature, with 1''H''-tetraphosphole having a more pyramidal geometry of the phosphorus at position 1. Instead, it is the anionic tetraphospholides that are aromatic. Strongly inductive effect, inductively electron-withd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Click Reaction
Click chemistry is an approach to chemical synthesis that emphasizes efficiency, simplicity, selectivity, and modularity in chemical processes used to join molecular building blocks. It includes both the development and use of "click reactions", a set of simple, biocompatible chemical reactions that meet specific criteria like high yield, fast reaction rates, and minimal byproducts. It was first fully described by K. Barry Sharpless, Hartmuth C. Kolb, and M. G. Finn of The Scripps Research Institute in 2001. The paper argued that synthetic chemistry could emulate the way nature constructs complex molecules, using efficient reactions to join together simple, non-toxic building blocks. The term "click chemistry" was coined in 1998 by Sharpless' wife, Jan Dueser, who found the simplicity of this approach to chemical synthesis akin to clicking together Lego blocks. In fact, the simplicity of click chemistry represented a paradigm shift in synthetic chemistry, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azides
In chemistry, azide (, ) is a Linear molecular geometry, linear, polyatomic anion with the Chemical formula, formula and Chemical structure, structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant application of azides is as a propellant in air bags. Preparation Sodium azide is made industrially by the reaction of nitrous oxide, with sodium amide in liquid ammonia as solvent: : Many inorganic azides can be prepared directly or indirectly from sodium azide. For example, lead azide, used in detonators, may be prepared from the Salt metathesis reaction, metathesis reaction between lead nitrate and sodium azide. An alternative route is direct reaction of the metal with silver azide dissolved in liquid ammonia. Some azides are produced by treating the carbonate Salt (chemistry), salts with hydrazoic acid. Bonding Azide is isoelectronic with carbon dioxide , cyanate , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an anion, an ion with negative charge. Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,.... Carboxylate esters have the general formula (also written as ), where R and R′ are organic groups. Synthesis Carboxylate ions can be formed by deprotonation of carboxylic acids. Such acids typically have p''K''a of less than 5, meaning that they can be deprotonated by many bases, such as sodium hydroxide or sodium bicarbonate. : Resonance stabilization of the carboxylate ion Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized by resonance. The negative charge that is left after deprotonation of the carboxyl group is delocalized between the two electronegat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acylation
In chemistry, acylation is a broad class of chemical reactions in which an acyl group () is added to a substrate. The compound providing the acyl group is called the acylating agent. The substrate to be acylated and the product include the following: *alcohols, esters * amines, amides * arenes or alkenes, ketones A particularly common type of acylation is acetylation, the addition of the acetyl group. Closely related to acylation is formylation, which employ sources of "HCO+ in place of "RCO+". Examples Because they form a strong electrophile when treated with Lewis acids, acyl halides are commonly used as acylating agents. For example, Friedel–Crafts acylation uses acetyl chloride () as the agent and aluminum chloride () as a catalyst to add an acetyl group to benzene: This reaction is an example of electrophilic aromatic substitution. Acyl halides and acid anhydrides of carboxylic acids are also common acylating agents. In some cases, active esters exhibit compa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
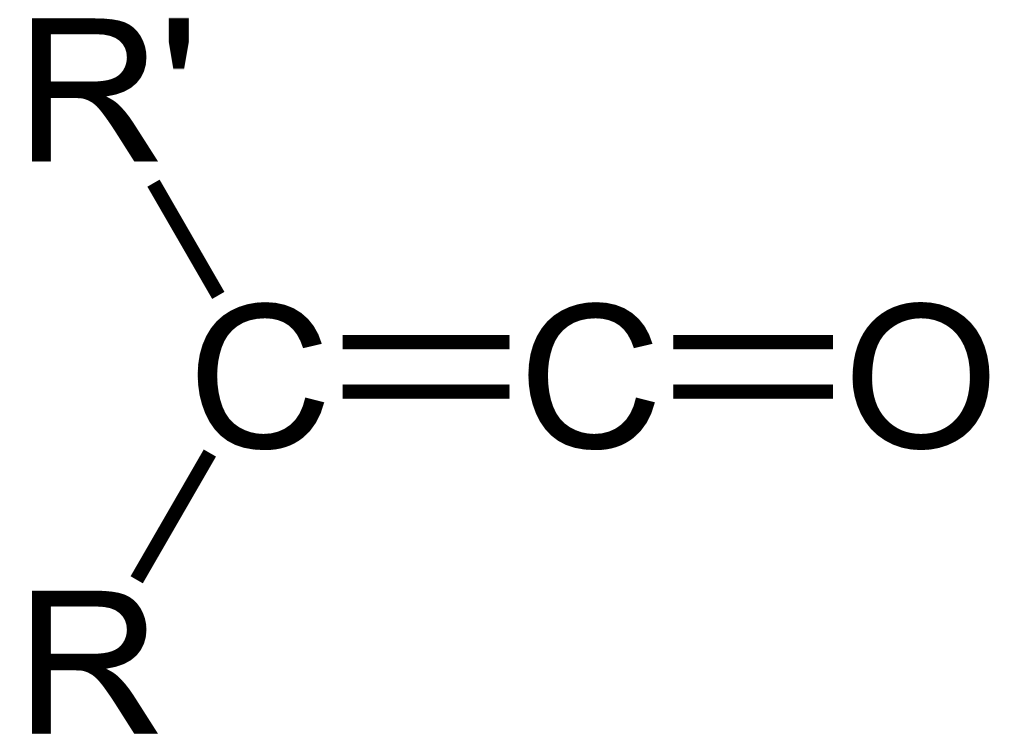
Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are chemical stability, unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocyanation
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst if the substrate alkene is unactivated. This conversion is conducted on an industrial scale for the production of precursors to nylon. Direct hydrocyanation is rare in the laboratory because hydrogen cyanide is extremely toxic, but transfer variants can allow other nitrilic compounds to serve as hydrogen cyanide synthons. Hydrocyanation of unactivated alkenes Industrially, hydrocyanation is commonly performed on alkenes catalyzed by nickel complexes of phosphite () ligands. A general reaction is shown:Piet W.N.M. van Leeuwen "Homogeneous Catalysis: Understanding the Art", 2004, Wiley-VCH, Weinheim. : Mechanism The reaction proceeds via oxidative addition of HCN to a low-valent metal complex to give a hydrido cyanide complex. Subsequently the alkene binds to the complex. The intermediate then undergoes m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldoxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides () with general structure . Oximes are usually generated by the reaction of hydroxylamine with aldehydes () or ketones (). The term ''oxime'' dates back to the 19th century, a combination of the words ''oxygen'' and ''imine''. Structure and properties If the two side-chains on the central carbon are different from each other—either an aldoxime, or a ketoxime with two different "R" groups—the oxime can often have two different geometric stereoisomeric forms according to the ''E''/''Z'' configuration. An older terminology of ''syn'' and ''anti'' was used to identify especially aldoximes according to whether the R group was closer or further from the hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydration Reaction
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of an H2O from the reacting molecule(s) or ion(s). This reaction results in the release of the H2O as water. When the reaction involves the coupling of two molecules into a single molecule it is referred to as a condensation reaction. Dehydration reactions are common processes in the manufacture of chemical compounds as well as naturally occurring within living organisms. The reverse of a dehydration reaction is called a hydration reaction. The reverse of a condensation reaction yielding water is called hydrolysis. Condensation reactions occurring in living organisms Condensation dehydration reactions are fundamental to the existence of life as this type of reaction produces proteins from amino acids, DNA and RNA from nucleotides, fats from fatty acids, and polysaccharides (eg. cellulose, starch, sugar, lactose) from monosaccharides (eg. glucose and fructose). The formation of the pyrophosphat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |