|
Acenes General Structure
In organic chemistry, the acenes or polyacenes are a class of organic compounds and polycyclic aromatic hydrocarbons made up of benzene () Ring (chemistry), rings which have been linearly fused-ring compound, fused. They follow the general molecular formula . The larger representatives have potential interest in optoelectronic applications and are actively researched in chemistry and electrical engineering. Pentacene has been incorporated into organic field-effect transistors, reaching charge carrier mobilities as high as 5 cm2/Vs. The first 5 unsubstituted members are listed in the following table: Hexacene is not stable in air, and Dimer (chemistry), dimerises upon isolation. Heptacene (and larger acenes) is very Reactivity (chemistry), reactive and has only been isolated in a matrix. However, bis(trialkylsilylethynylated) versions of heptacene have been isolated as crystalline solids. Larger acenes Due to their increased conjugated system, conjugation length the larg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azene
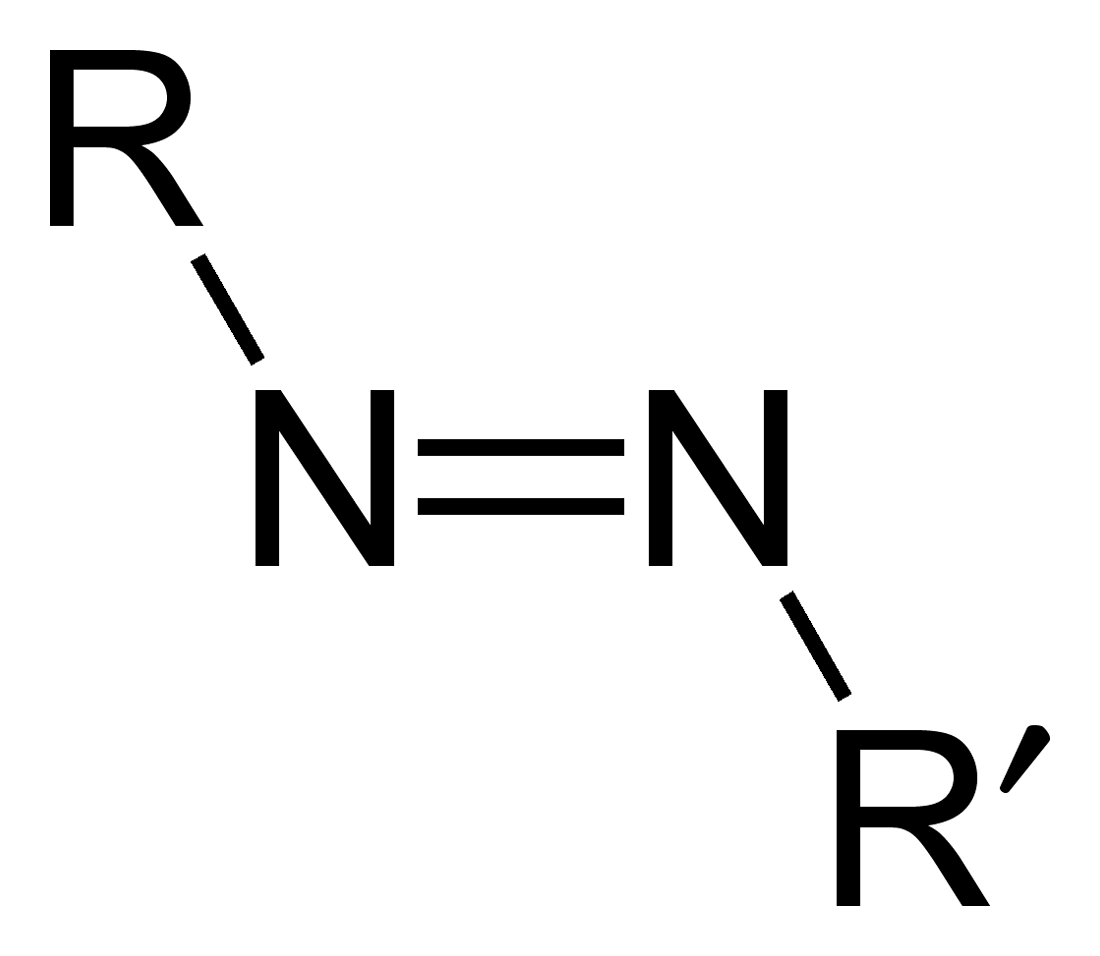
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene.", where Ph stands for phenyl group. The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds urinary tract infections">Phenazopyridine, an aryl azo compound, is used to treat urinary tract infections">150px Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the Cis-trans isomerism, ''trans'' isomer, but upon illumination, converts to the Cis-trans isomerism, ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic subst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diradical
In chemistry, a diradical is a chemical species, molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate energy level, degenerate. The term "diradical" is mainly used to describe organic compounds, where most diradicals are extremely reactivity (chemistry), reactive and non-Kekulé molecules that are rarely isolated. Diradicals are even-electron molecules but have one fewer chemical bond, bond than the number permitted by the octet rule. Examples of diradical species can also be found in coordination chemistry, for example among metal dithiolene complex, bis(1,2-dithiolene) metal complexes. Spin states Diradicals are usually triplet state, triplets. The phrases ''singlet'' and ''triplet'' are derived from the multiplicity of states of diradicals in electron spin resonance: a singlet diradical has one state (S=0, Ms=2*0+1=1, ms=0) and exhibits no signal in electron paramagnetic resonance, EPR and a triplet diradical has 3 states (S=1, Ms=2*1+1= ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating the presence of double bonds within the carbon structure. Graphene is known for its exceptionally high Ultimate tensile strength, tensile strength, Electrical resistivity and conductivity, electrical conductivity, Transparency and translucency, transparency, and being the thinnest two-dimensional material in the world. Despite the nearly transparent nature of a single graphene sheet, graphite (formed from stacked layers of graphene) appears black because it absorbs all visible light wavelengths. On a microscopic scale, graphene is the strongest material ever measured. The existence of graphene was first theorized in 1947 by P. R. Wallace, Philip R. Wallace during his research on graphite's electronic properties, while the term ''graphen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Nanotube
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range ( nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized: * ''Single-walled carbon nanotubes'' (''SWCNTs'') have diameters around 0.5–2.0 nanometres, about 100,000 times smaller than the width of a human hair. They can be idealised as cutouts from a two-dimensional graphene sheet rolled up to form a hollow cylinder. * ''Multi-walled carbon nanotubes'' (''MWCNTs'') consist of nested single-wall carbon nanotubes in a nested, tube-in-tube structure. Double- and triple-walled carbon nanotubes are special cases of MWCNT. Carbon nanotubes can exhibit remarkable properties, such as exceptional tensile strength and thermal conductivity because of their nanostructure and strength of the bonds between carbon atoms. Some SWCNT structures exhibit high electrical conductivity while others are semiconductors. In addition, carbon nanotubes can b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugated System
In physical organic chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases Chemical stability, stability. It is Resonance (chemistry), conventionally represented as having alternating single and multiple covalent bond, bonds. Lone pairs, radical (chemistry), radicals or carbenium ions may be part of the system, which may be Cyclic molecule, cyclic, acyclic, Linear molecular geometry, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele (chemist), Johannes Thiele. Conjugation is the orbital overlap, overlap of one p-orbital with another across an adjacent Sigma bond, σ bond (in transition metals, d-orbitals can be involved). A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond. They allow a delocalization of pi el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactivity (chemistry)
In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy. ''Reactivity'' refers to: * the chemical reactions of a single substance, * the chemical reactions of two or more substances that interact with each other, * the systematic study of sets of reactions of these two kinds, * methodology that applies to the study of reactivity of chemicals of all kinds, * experimental methods that are used to observe these processes, and * theories to predict and to account for these processes. The chemical reactivity of a single substance (reactant) covers its behavior in which it: * decomposes, * forms new substances by addition of atoms from another reactant or reactants, and * interacts with two or more other reactants to form two or more products. The chemical reactivity of a substance can refer to the variety of circumstances (conditions that include temperature, pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
In chemistry, dimerization is the process of joining two identical or similar Molecular entity, molecular entities by Chemical bond, bonds. The resulting bonds can be either strong or weak. Many symmetrical chemical species are described as dimers, even when the monomer is unknown or highly unstable. The term ''homodimer'' is used when the two subunits are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called Dissociation (chemistry), dissociation. When two oppositely-charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Danish chemist Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Many OH-containing molecules form dimers, e.g. the water dimer. Dimers that form based on w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heptacene 200
Heptacene is an organic compound and a polycyclic aromatic hydrocarbon and the seventh member of the acene or polyacene family of linear fused benzene rings. This compound has long been pursued by chemists because of its potential interest in electronic applications and was first synthesized but not cleanly isolated in 2006. Heptacene was finally fully characterized in bulk by researchers in Germany and the United States in 2017. : The final step is a photochemical decarbonylization with a 1,2-dione bridge extruded as carbon monoxide. In solution heptacene is not formed because it is very unstable being a reactive DA diene and quickly reacts with oxygen or forms dimers. When on the other hand the dione precursor is dissolved in a PMMA matrix first, heptacene can be studied by spectroscopy. Heptacene has been studied spectroscopically at cryogenic temperatures in a matrix. When dissolved in sulfuric acid the heptacene dication is reported to be stable at room-temperature for more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexacene 200
Hexacene is an aromatic compound consisting of six linearly-fused benzene rings. It is a blue-green, air-stable solid with low solubility. Hexacene is one of a series of linear polycyclic molecules created by such aromatic ring fusions, a series termed acenes; the previous in the series is pentacene (with five fused rings) and the next is heptacene (with seven). It and other acenes and their derivatives have been investigated in potential applications related to organic semiconductors. Like larger acenes, hexacene is poorly soluble, but derivatives have been prepared with improved solubility, such as 6,15-Bis(tri-t-butylsilylethynyl)hexacene, which melts with decomposition at 96 °C. Syntheses and structure Hexacene has been the subject of many syntheses. One route uses thermal decarbonylation of a monoketone precursor. Further reading *First synthesis: **Marschalk, C. Linear hexacenes. Bull. Soc. Chim. Fr. 6, 1112–1121 (1939). ** ** *By dehydrogenation of ''hexacosahyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |