|
Absolute Zero
Absolute zero is the lowest possible temperature, a state at which a system's internal energy, and in ideal cases entropy, reach their minimum values. The absolute zero is defined as 0 K on the Kelvin scale, equivalent to −273.15 °C on the Celsius, Celsius scale, and −459.67 °F on the Fahrenheit scale. The Kelvin and Rankine temperature scales set their zero points at absolute zero by design. This limit can be estimated by extrapolating the ideal gas law to the temperature at which the volume or pressure of a classical gas becomes zero. At absolute zero, there is no thermal motion. However, due to quantum mechanics, quantum effects, the particles still exhibit minimal motion mandated by the Uncertainty principle, Heisenberg uncertainty principle and, for a system of fermions, the Pauli exclusion principle. Even if absolute zero could be achieved, this residual quantum motion would persist. Although absolute zero can be approached, it cannot be reached. Som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), with the third being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microstate (statistical Mechanics)
In statistical mechanics, a microstate is a specific configuration of a system that describes the precise positions and momenta of all the individual particles or components that make up the system. Each microstate has a certain probability of occurring during the course of the system's thermal fluctuations. In contrast, the macrostate of a system refers to its macroscopic properties, such as its temperature, pressure, volume and density. Treatments on statistical mechanics define a macrostate as follows: a particular set of values of energy, the number of particles, and the volume of an isolated thermodynamic system is said to specify a particular macrostate of it. In this description, microstates appear as different possible ways the system can achieve a particular macrostate. A macrostate is characterized by a probability distribution of possible states across a certain statistical ensemble of all microstates. This distribution describes the probability of finding the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Can T=0 Be Reached
Can may refer to: Language * A verb for ability * A verb for probability Containers * A container used for food preservation in canning ** Aluminum can ** Drink can ** Steel and tin cans * Trash can * Oil can * Petrol can Music * Can (band), West Germany, 1968 ** ''Can'' (album), 1979 * Can (South Korean band) Abbreviations *Canada, a country *Cantoris, side of a church or choir Other * Can (name), Turkish and Circassian given name and surname * Can (verb) * Canning of food * River Can, Essex, UK * Tomato can (sports idiom) See also * CAN (other) * Cann (other) * Cans (other) * Kan (other) Kan or KAN may refer to: Places * Kan (river), a tributary of the Yenisey in Russia * Kan District of Iran * Kan, Kyrgyzstan, a village in Batken Region * Mallam Aminu Kano International Airport, Kano, Nigeria, IATA code * Kannapolis (Amtrak ... * Can-can (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the molar gas constant, in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating Johnson–Nyquist noise, thermal noise in resistors. The Boltzmann constant has Dimensional analysis, dimensions of energy divided by temperature, the same as entropy and heat capacity. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 revision of the SI, the Boltzmann constant is one of the seven "Physical constant, defining constants" that have been defined so as to have exact finite decimal values in SI units. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly joules per kelvin, with the effect of defining the SI unit ke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
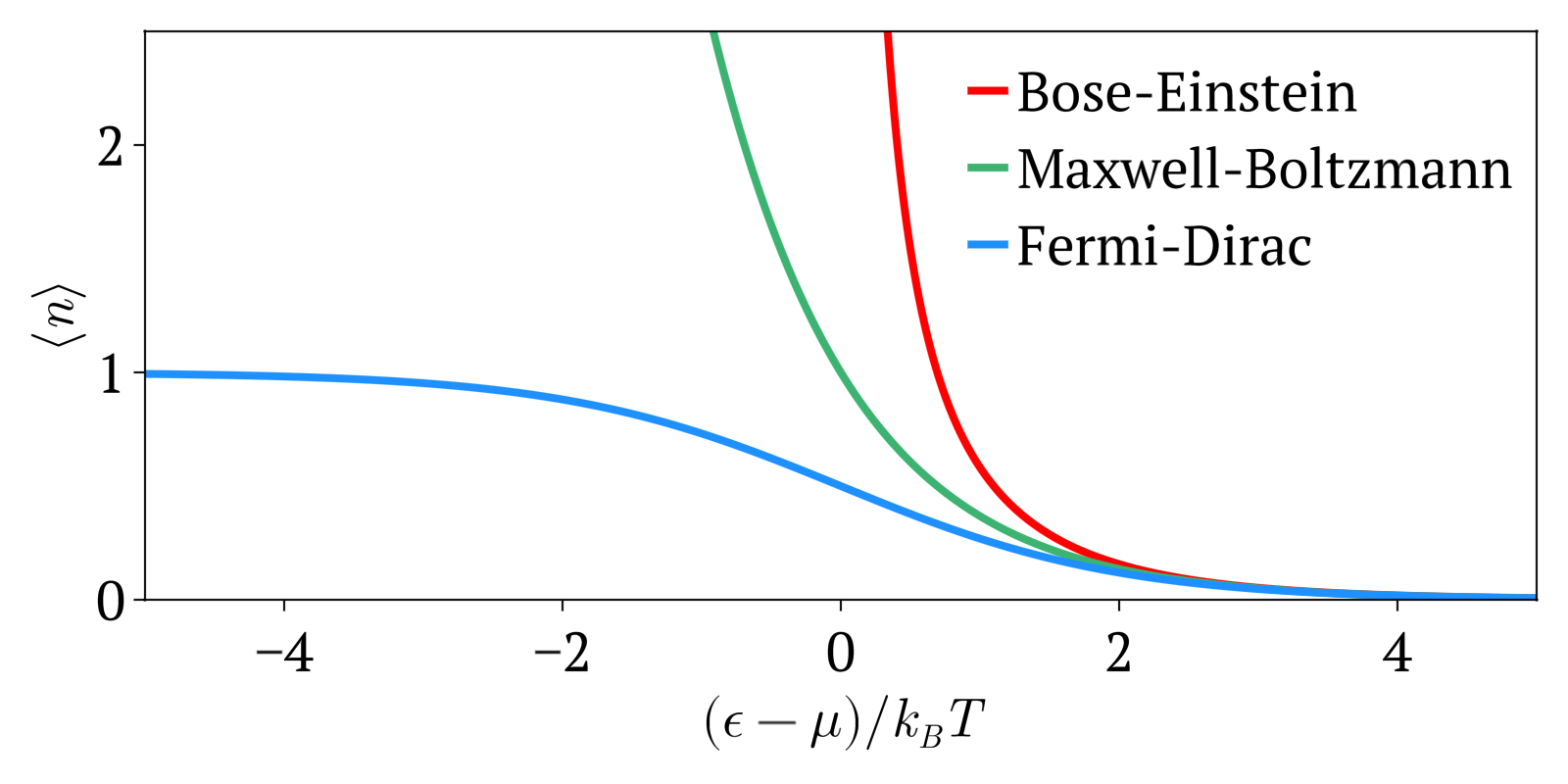
Bose–Einstein Statistics
In quantum statistics, Bose–Einstein statistics (B–E statistics) describes one of two possible ways in which a collection of non-interacting identical particles may occupy a set of available discrete energy states at thermodynamic equilibrium. The aggregation of particles in the same state, which is a characteristic of particles obeying Bose–Einstein statistics, accounts for the cohesive streaming of laser light and the frictionless creeping of superfluid helium. The theory of this behaviour was developed (1924–25) by Satyendra Nath Bose, who recognized that a collection of identical and indistinguishable particles could be distributed in this way. The idea was later adopted and extended by Albert Einstein in collaboration with Bose. Bose–Einstein statistics apply only to particles that do not follow the Pauli exclusion principle restrictions. Particles that follow Bose-Einstein statistics are called bosons, which have integer values of spin. In contrast, particles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi–Dirac Statistics
Fermi–Dirac statistics is a type of quantum statistics that applies to the physics of a system consisting of many non-interacting, identical particles that obey the Pauli exclusion principle. A result is the Fermi–Dirac distribution of particles over energy states. It is named after Enrico Fermi and Paul Dirac, each of whom derived the distribution independently in 1926. Fermi–Dirac statistics is a part of the field of statistical mechanics and uses the principles of quantum mechanics. Fermi–Dirac statistics applies to identical and indistinguishable particles with half-integer spin (1/2, 3/2, etc.), called fermions, in thermodynamic equilibrium. For the case of negligible interaction between particles, the system can be described in terms of single-particle energy states. A result is the Fermi–Dirac distribution of particles over these states where no two particles can occupy the same state, which has a considerable effect on the properties of the system. Fermi� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maxwell–Boltzmann Statistics
In statistical mechanics, Maxwell–Boltzmann statistics describes the distribution of classical material particles over various energy states in thermal equilibrium. It is applicable when the temperature is high enough or the particle density is low enough to render quantum effects negligible. The expected number of particles with energy \varepsilon_i for Maxwell–Boltzmann statistics is : \langle N_i \rangle = \frac = \frac\,g_i e^, where: * \varepsilon_i is the energy of the ''i''th energy level, * \langle N_i \rangle is the average number of particles in the set of states with energy \varepsilon_i, * g_i is the degeneracy of energy level ''i'', that is, the number of states with energy \varepsilon_i which may nevertheless be distinguished from each other by some other means,For example, two simple point particles may have the same energy, but different momentum vectors. They may be distinguished from each other on this basis, and the degeneracy will be the number of po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. Sometimes called statistical physics or statistical thermodynamics, its applications include many problems in a wide variety of fields such as biology, neuroscience, computer science Computer science is the study of computation, information, and automation. Computer science spans Theoretical computer science, theoretical disciplines (such as algorithms, theory of computation, and information theory) to Applied science, ..., information theory and sociology. Its main purpose is to clarify the properties of matter in aggregate, in terms of physical laws governing atomic motion. Statistical mechanics arose out of the development of classical thermodynamics, a field for which it was successful in explaining macroscopic physical properties—such as temperature, pressure, and heat capacity—in terms of microscop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fahrenheit
The Fahrenheit scale () is a scale of temperature, temperature scale based on one proposed in 1724 by the German-Polish physicist Daniel Gabriel Fahrenheit (1686–1736). It uses the degree Fahrenheit (symbol: °F) as the unit. Several accounts of how he originally defined his scale exist, but the original paper suggests the lower defining point, 0 °F, was established as the freezing temperature of a solution of brine made from a mixture of water, ice, and ammonium chloride (a Salt (chemistry), salt). The other limit established was his best estimate of the average human body temperature, originally set at 90 °F, then 96 °F (about 2.6 °F less than the modern value due to a later redefinition of the scale). For much of the 20th century, the Fahrenheit scale was defined by two fixed points with a 180 °F separation: the temperature at which pure water freezes was defined as 32 °F and the boiling point of water was defined to be 212 °F, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rankine Scale
The Rankine scale ( ) is an absolute scale of thermodynamic temperature named after the University of Glasgow engineer and physicist Macquorn Rankine, who proposed it in 1859. History Similar to the Kelvin scale, which was first proposed in 1848, zero on the Rankine scale is absolute zero, but a temperature difference of one Rankine degree (°R or °Ra) is defined as equal to one Fahrenheit degree, rather than the Celsius degree used on the Kelvin scale. In converting from kelvin to degrees Rankine, 1 K = °Ra or 1 K = 1.8 °Ra. A temperature of 0 K (−273.15 °C; −459.67 °F) is equal to 0 °Ra.B.8 Factors for Units Listed Alphabetically from Usage The Rankine scale is used in engineering systems where ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kelvin
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By definition, the Celsius scale (symbol °C) and the Kelvin scale have the exact same magnitude; that is, a rise of 1 K is equal to a rise of 1 °C and vice versa, and any temperature in degrees Celsius can be converted to kelvin by adding 273.15. The 19th century British scientist Lord Kelvin first developed and proposed the scale. It was often called the "absolute Celsius" scale in the early 20th century. The kelvin was formally added to the International System of Units in 1954, defining 273.16 K to be the triple point of water. The Celsius, Fahrenheit, and Rankine scales were redefined in terms of the Kelvin scale using this definition. The 2019 revision of the SI now defines the kelvin in terms of energy by setting the Bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |