|
ATC Code D11
D11A Other dermatological preparations D11AA Antihidrotics :D11AA01 Glycopyrronium D11AC Medicated shampoos :D11AC01 Cetrimide :D11AC02 Cadmium compounds :D11AC03 Selenium compounds :D11AC06 Povidone-iodine :D11AC08 Sulfur compounds :D11AC09 Xenysalate :D11AC30 Others D11AE Androgens for topical use :D11AE01 Metandienone D11AF Wart and anti-corn preparations :''Empty group'' D11AH Agents for dermatitis, excluding corticosteroids :D11AH01 Tacrolimus :D11AH02 Pimecrolimus :D11AH03 Cromoglicic acid :D11AH04 Alitretinoin :D11AH05 Dupilumab :D11AH06 Crisaborole :D11AH07 Tralokinumab :D11AH08 Abrocitinib :D11AH09 Ruxolitinib :QD11AH90 Oclacitinib :QD11AH91 Lokivetmab D11AX Other dermatologicals :D11AX01 Minoxidil :D11AX02 Gamolenic acid :D11AX03 Calcium gluconate :D11AX04 Lithium succinate :D11AX05 Magnesium sulfate :D11AX06 Mequinol :D11AX08 Tiratricol :D11AX09 Oxaceprol :D11AX10 Finasteride :D11AX11 Hydroquinone :D11AX12 Pyrithione zinc :D11AX13 Monobenzone :D11AX16 Eflornithi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Cromoglicic Acid
Cromoglicic acid (INN)—also referred to as cromolyn ( USAN), cromoglycate (former BAN), or cromoglicate—is traditionally described as a mast cell stabilizer, and is commonly marketed as the sodium salt sodium cromoglicate or cromolyn sodium. This drug prevents the release of inflammatory chemicals such as histamine from mast cells. Cromoglicic acid has been the non-corticosteroid treatment of choice in the treatment of asthma, for which it has largely been replaced by leukotriene receptor antagonists because of their convenience (and perceived safety). Cromoglicic acid requires administration four times daily, and does not provide additive benefit in combination with inhaled corticosteroids. History Cromolyn sodium was discovered in 1965 by Roger Altounyan, a pharmacologist who had asthma. It is considered a breakthrough drug in management of asthma, as the patients can be freed from steroids in many cases; however, it is mainly effective as a prophylaxis for allergic and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
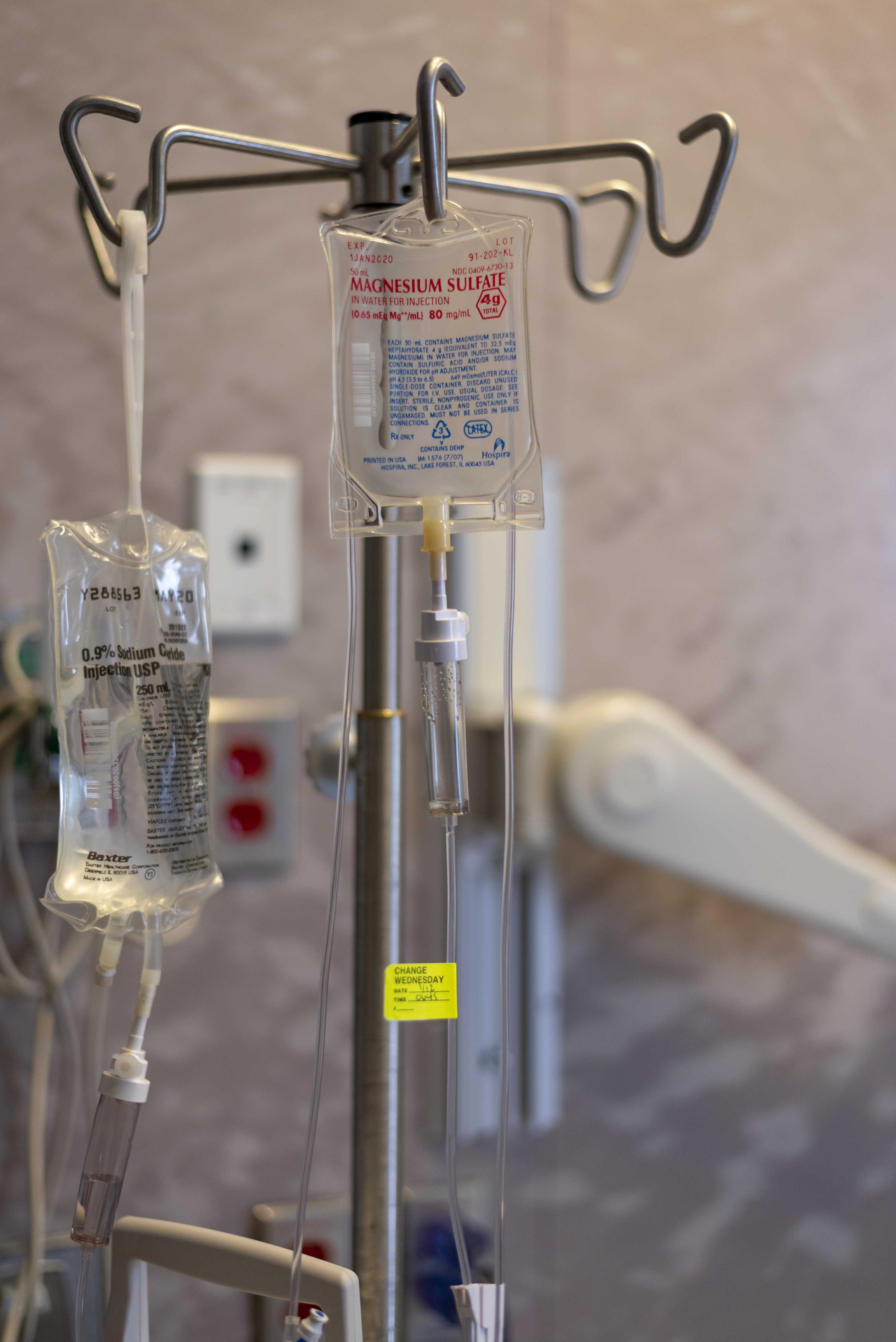 |
Magnesium Sulfate (medical Use)
Magnesium sulfate as a medication is used to treat and prevent low blood magnesium and seizures in women with eclampsia. It is also used in the treatment of torsades de pointes, severe asthma exacerbations, constipation, and barium poisoning. It is given by injection into a vein or muscle as well as by mouth. As epsom salts, it is also used for mineral baths. Common side effects include low blood pressure, skin flushing, and low blood calcium. Other side effects may include vomiting, muscle weakness, and decreased breathing. While there is evidence that use during pregnancy may harm the baby, the benefits in certain conditions are greater than the risks. Its use during breastfeeding is deemed to be safe. The way it works is not fully understood, but is believed to involve depressing the action of neurons. Magnesium sulfate came into medical use at least as early as 1618. It is on the World Health Organization's List of Essential Medicines. Forms Magnesium sulfate i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Lithium Succinate
Lithium succinate (C4H4Li2O4), the dilithium salt of succinic acid, is a drug used in the treatment of seborrhoeic dermatitis and proposed for the treatment of anogenital warts Genital warts are a sexually transmitted infection caused by certain types of human papillomavirus (HPV). They are generally pink in color and project out from the surface of the skin. Usually they cause few symptoms, but can occasionally be pa .... References External links * Lithium salts Organolithium compounds Succinates {{dermatologic-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Calcium Gluconate
Calcium gluconate is a mineral supplement and medication. As a medication it is used by injection into a vein to treat low blood calcium, high blood potassium, and magnesium toxicity. Supplementation is generally only required when there is not enough calcium in the diet. Supplementation may be done to treat or prevent osteoporosis or rickets. It can also be taken by mouth but is not recommended for injection into a muscle. Side effects when injected include slow heart rate, pain at the site of injection, and low blood pressure. When taken by mouth side effects may include constipation and nausea. Blood calcium levels should be measured when used and extra care should be taken in those with a history of kidney stones. At normal doses, use is regarded as safe in pregnancy and breastfeeding. Calcium gluconate is made by mixing gluconic acid with calcium carbonate or calcium hydroxide. Calcium gluconate came into medical use in the 1920s. It is on the World Health Organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Minoxidil
Minoxidil, sold under the brand name Rogaine among others, is a medication used for the treatment of high blood pressure and pattern hair loss. It is an antihypertensive vasodilator. It is available as a generic medication by prescription in oral tablet form and over the counter as a topical liquid or foam. Medical uses Minoxidil, when used for hypertension, is generally reserved for use in severe hypertension patients who can not respond to at least two agents and a diuretic. Minoxidil is also generally administered with a loop diuretic to prevent sodium and potassium retention. It may also cause a reflex tachycardia and thus is prescribed with a beta blocker. Minoxidil, applied topically, is widely used for the treatment of hair loss. It is effective in helping promote hair growth in people with androgenic alopecia regardless of sex. Minoxidil must be used indefinitely for continued support of existing hair follicles and the maintenance of any experienced hair regrowt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Lokivetmab
Lokivetmab, trade name Cytopoint, is a monoclonal antibody used to treat atopic dermatitis in dogs. It acts against interleukin 31 (IL-31), which is a cytokine involved in causing itchiness (pruritus). Lokivetmab is administered by subcutaneous injection; each dose is effective for four to eight weeks. The United States Department of Agriculture (USDA) approved lokivetmab (manufactured by Zoetis Zoetis Inc. (/zō-EH-tis/) is an American drug company, the world's largest producer of medicine and vaccinations for pets and livestock. The company was a subsidiary of Pfizer, the world's largest drug maker, but with Pfizer's spinoff of its 83 ... and sold under the trade name Cytopoint) in December 2016, and it was approved by the European Medicines Agency in 2017. Lokivetmab was the first monoclonal antibody to be approved for use in animals in the European Union. References External links * Monoclonal antibodies Dog medications {{dermatologic-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Oclacitinib
Oclacitinib (brand name Apoquel) is a veterinary medication used in the control of atopic dermatitis and pruritus from allergic dermatitis in dogs at least 12 months of age. Chemically, it is a synthetic cyclohexylamino pyrrolopyrimidine janus kinase inhibitor that is relatively selective for JAK1. It inhibits signal transduction when the JAK is activated and thus helps downregulate expression of inflammatory cytokines. While oclacitinib is effective, its long-term safety is currently unknown. Oclacitinib was approved by the FDA in 2013. Uses Oclacitinib is labeled to treat atopic dermatitis and itchiness (pruritus) caused by allergies in dogs, though it has also been used to reduce the itchiness and dermatitis caused by flea infestations. It is considered to be highly effective in dogs, and has been established as safe for at least short-term use. Its efficacy equals that of prednisolone at first, though oclacitinib has been found to be more effective in the short term ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Ruxolitinib
Ruxolitinib, sold under the brand names Jakafi and Jakavi, is a medication used for the treatment of intermediate or high-risk myelofibrosis, a type of myeloproliferative disorder that affects the bone marrow; polycythemia vera (PCV), when there has been an inadequate response to or intolerance of hydroxyurea; and steroid-refractory acute graft-versus-host disease. Ruxolitinib is a Janus kinase inhibitor. It was developed and marketed by Incyte Corp in the US under the brand name Jakafi, and by Novartis elsewhere in the world, under the brand name Jakavi. It was approved for medical use in the United States in 2011, and in the European Union in 2012. Ruxolitinib is the first FDA-approved pharmacologic treatment to address repigmentation in vitiligo patients. Medical uses In the United States and the European Union, ruxolitinib is indicated for the treatment of disease-related splenomegaly or symptoms in adults with primary myelofibrosis (also known as chronic idiopathic myel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Abrocitinib
Abrocitinib, sold under the brand name Cibinqo, is a medication used for the treatment of atopic dermatitis (eczema). It is a Janus kinase inhibitor and it was developed by Pfizer. It is taken by mouth. The most common side effects include nausea (feeling sick), headache, acne, herpes simplex (viral infection of the mouth or the genitals), increased levels of creatine phosphokinase in the blood (an enzyme released into the blood when muscle is damaged), vomiting, dizziness and pain in the upper belly. Abrocitinib was approved for medical use in the European Union in December 2021, and in the United States in January 2022. Medical uses In the EU, abrocitinib is indicated In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedure or medication. An indication can commonly be confused with the term diagnosis. A diagnosis ... for the treatment of moderate-to-severe atopic derm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Tralokinumab
Tralokinumab sold under the brand names Adtralza (EU/UK) and Adbry (US) among others, is a human monoclonal antibody used for the treatment of atopic dermatitis. Tralokinumab targets the cytokine interleukin 13. The most common side effects include upper respiratory tract infections (colds and other infections of the nose and throat), reactions at the injection site, and redness and discomfort in the eye. Tralokinumab was approved for medical use in the European Union and in the United Kingdom in June 2021. It was approved for medical use in the United States in December 2021. The U.S. Food and Drug Administration The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ... (FDA) considers it to be a first-in-class medication. Medical uses Tralokinumab is indicated for the treatment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |