|
AMPH-CR
Tetrahydroisoquinoline (TIQ or THIQ), also known as AMPH-CR, is an organic compound with the chemical formula C9H11N. Classified as a secondary amine, it is derived from isoquinoline by hydrogenation. It is a colorless viscous liquid that is miscible with most organic solvents. The tetrahydroisoquinoline skeleton is encountered in a number of bioactive compounds and drugs. Pharmacology THIQ is a conformationally restrained (CR) or cyclized analogue of β-phenethylamine and amphetamine and is also known as AMPH-CR. In contrast to amphetamine however, THIQ fails to substitute for dextroamphetamine in rodent drug discrimination tests, suggesting that it lacks stimulant effects. Similar findings have been made for other tetrahydroisoquinoline analogues of psychoactive phenethylamines, for instance DOM-CR. In any case, THIQ does substitute for TDIQ (MDTIQ), a selective α2-adrenergic receptor ligand, indicating that it is not pharmacologically inactive. Reactions As a secondar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DOM-CR
DOM-CR, or DOM/CR, an acronym of "DOM-conformationally restrained", is a tetrahydroisoquinoline (THIQ) and cyclized phenethylamine related to the psychedelics DOM and 2C-D. It is a cyclized THIQ analogue of DOM and 2C-D. DOM-CR shows more than 20-fold reduced affinity for the serotonin 5-HT2A receptor compared to DOM (Ki = 2,150nM vs. 100nM, respectively). In contrast to DOM, DOM-CR does not substitute for DOM in rodent drug discrimination tests, suggesting that it lacks psychedelic effects. Similarly, DOM-CR does not substitute for dextroamphetamine or MDMA, suggesting that it likewise lacks stimulant or entactogenic effects. However, DOM-CR does substitute for TDIQ (MDTHIQ), a selective α2-adrenergic receptor ligand. At high doses, DOM-CR produces behavioral disruption in drug discrimination tests. In contrast to DOM and amphetamine, DOM-CR does not produce hyperlocomotion in rodents. DOM-CR was first described in the scientific literature by Richard Glennon and colleag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TDIQ
TDIQ, also known as MDTHIQ or MDA-CR, is a tetrahydroisoquinoline drug used in scientific research, which has anxiolytic and anorectic effects in animals. It has an unusual effects profile in animals, with the effects generalising to cocaine and partially to MDMA and ephedrine, but the effects did not generalise to amphetamine and TDIQ does not have any stimulant effects. It is thought these effects are mediated via a partial agonist action at Alpha-2 adrenergic receptors, and TDIQ has been suggested as a possible drug for the treatment of cocaine dependence. See also * Substituted tetrahydroisoquinoline * AMPH-CR and DOM-CR * MDAI * MDAT * Norsalsolinol * Tetrahydroisoquinoline Tetrahydroisoquinoline (TIQ or THIQ), also known as AMPH-CR, is an organic compound with the chemical formula C9H11N. Classified as a secondary amine, it is derived from isoquinoline by hydrogenation. It is a colorless viscous liquid that is mis ... * C10H11NO2 References Alpha-2 adrenergic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decahydroisoquinoline
Decahydroisoquinoline is a nitrogen-containing heterocycle with the chemical formula . It is the saturated form of isoquinoline. Decahydroisoquinoline can be formed by the hydrogenation of isoquinoline or tetrahydroisoquinoline. Isomers There are four stereoisomers of decahydroisoquinoline which differ by the configuration of the two carbon atoms at the ring fusion: Occurrence The decahydroisoquinoline occurs naturally in some alkaloids, including gephyrotoxins and pumiliotoxin C which are found in amphibian skins. A variety of pharmaceutical drugs include a decahydroisoquinoline ring system within their structure, including ciprefadol, dasolampanel, nelfinavir, saquinavir, and tezampanel Tezampanel (, ) (developmental code names LY-293,558, LY-326,325, NGX-424) is a drug originally developed by Eli Lilly which acts as a competitive antagonist of the AMPA and kainate subtypes of the ionotropic glutamate receptor family, with sel .... References {{reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diclofensine
Diclofensine (Ro 8-4650) was developed by Hoffmann-La Roche in the 1970s in the search for a new antidepressant. It was found that the (''S'')-isomer was responsible for activity. Diclofensine is a stimulant drug which acts as a triple monoamine reuptake inhibitor, primarily inhibiting the reuptake of dopamine and norepinephrine, with affinities ( Ki) of 16.8 nM, 15.7 nM, and 51 nM for DAT, NET, and SERT (dopamine, norepinephrine and serotonin transporters), respectively. It was found to be an effective antidepressant in human trials, with relatively few side effects, but was ultimately dropped from clinical development, possibly due to concerns about its abuse potential. Diclofensine is chemically a tetrahydroisoquinoline (THIQ) derivative, as is nomifensine. Synthesis The condensation of m-anisaldehyde 91-31-1(1) with methylamine gives N-methyl-3-methoxybenzenemethanimine 6928-30-6 Reduction of this Schiff-base intermediate with sodium borohydride gives (3-methoxybenzyl) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Medicinal Chemistry
The ''Journal of Medicinal Chemistry'' is a biweekly peer-reviewed medical journal covering research in medicinal chemistry. It is published by the American Chemical Society. It was established in 1959 as the ''Journal of Medicinal and Pharmaceutical Chemistry'' and obtained its current name in 1963. Philip S. Portoghese served as editor-in-chief from 1972 to 2011. In 2012, Gunda Georg (University of Minnesota) and Shaomeng Wang (University of Michigan) succeeded Portoghese (University of Minnesota). In 2021, Craig W. Lindsley (Vanderbilt University) became editor-in-chief. According to the ''Journal Citation Reports'', the journal has a 2023 impact factor of 7.1. See also *ACS Medicinal Chemistry Letters ''ACS Medicinal Chemistry Letters'' is a monthly peer-reviewed scientific journal covering medicinal chemistry. It was established in 2009 and is published by the American Chemical Society. The editor-in-chief is Dennis C. Liotta (Emory University ... References External ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nomifensine
Nomifensine, formerly sold under the brand names Merital and Alival, is a norepinephrine–dopamine reuptake inhibitor (NDRI) drug that was developed in the 1960s by Hoechst AG (now Sanofi-Aventis), who then test marketed it in the United States. Nomifensine was considered an effective antidepressant that lacked sedative effects. It did not interact significantly with alcohol (drug), alcohol and lacked anticholinergic effects. No Drug withdrawal, withdrawal symptoms were seen after 6 months treatment. The drug was, however, considered not suitable for agitated patients as it presumably made agitation worse. In January 1986 the drug was withdrawn by its manufacturers for safety reasons. Some case reports in the 1980s suggested that there was potential for psychological dependence on nomifensine, typically in patients with a history of stimulant addiction, or when the drug was used in very high doses (400–600 mg per day). In a 1989 study it was investigated for use in tre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Ammonium Muscle Relaxants
The Quaternary ( ) is the current and most recent of the three periods of the Cenozoic Era in the geologic time scale of the International Commission on Stratigraphy (ICS), as well as the current and most recent of the twelve periods of the Phanerozoic eon. It follows the Neogene Period and spans from 2.58 million years ago to the present. The Quaternary Period is divided into two epochs: the Pleistocene (2.58 million years ago to 11.7 thousand years ago) and the Holocene (11.7 thousand years ago to today); a proposed third epoch, the Anthropocene, was rejected in 2024 by IUGS, the governing body of the ICS. The Quaternary is typically defined by the Quaternary glaciation, the cyclic growth and decay of continental ice sheets related to the Milankovitch cycles and the associated climate and environmental changes that they caused. Research history In 1759 Giovanni Arduino proposed that the geological strata of northern Italy could be divided into four successive for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
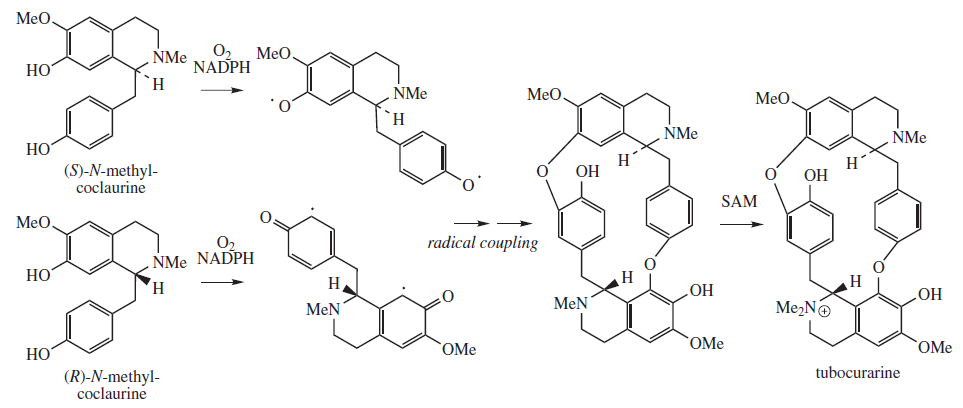
Tubocurarine
Tubocurarine (also known as ''d''-tubocurarine or DTC) is a toxic benzylisoquinoline alkaloid historically known for its use as an arrow poison. In the mid-1900s, it was used in conjunction with an anesthetic to provide skeletal muscle relaxation during surgery or mechanical ventilation. Safer alternatives, such as cisatracurium and rocuronium, have largely replaced it as an adjunct for clinical anesthesia and it is now rarely used. The specific form used was tubocurarine chloride, its hydrated hydrochloride salt. History Tubocurarine is a naturally occurring mono-quaternary alkaloid obtained from the bark of the Menispermaceous South American plant '' Chondrodendron tomentosum'', a climbing vine known to the European world since the Spanish conquest of South America. Curare had been used as a source of arrow poison by South American natives to hunt animals, and they were able to eat the animals' contaminated flesh subsequently without any adverse effects because tubocur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemical Pharmacology (journal)
''Biochemical Pharmacology'' is a peer-reviewed medical journal published by Elsevier. It covers research on the pharmacodynamics and pharmacokinetics of drugs and non-therapeutic xenobiotics. The editor-in-chief is Jacques Piette, University of Liege. , accessed on February 11th, 2013 Abstracting and indexing The journal is abstracted and indexed in: According to the '''', the journal received a 2019impact factor
[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parkinson's Disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor system, motor and non-motor systems. Symptoms typically develop gradually and non-motor issues become more prevalent as the disease progresses. The motor symptoms are collectively called parkinsonism and include tremors, bradykinesia, spasticity, rigidity as well as postural instability (i.e., difficulty maintaining balance). Non-motor symptoms develop later in the disease and include behavior change (individual), behavioral changes or mental disorder, neuropsychiatric problems such as sleep abnormalities, psychosis, anosmia, and mood swings. Most Parkinson's disease cases are idiopathic disease, idiopathic, though contributing factors have been identified. Pathophysiology involves progressive nerve cell death, degeneration of nerve cells in the substantia nigra, a midbrain region that provides dopamine to the basal ganglia, a system invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norsalsolinol
Norsalsolinol is a tetrahydroisoquinoline that is produced naturally in the body through the metabolism of dopamine. It has been shown to be a selective dopaminergic neurotoxin, and has been suggested as a possible cause of neurodegenerative conditions such as Parkinson's disease and the brain damage associated with alcoholism, although evidence for a causal relationship is unclear. The related compound (''R'')-salsolinol, which has been shown to be a product of ethanol metabolism, stereospecifically induces behavioral sensitization and leads to excessive alcohol intake in rats. See also * Substituted tetrahydroisoquinoline * 6-Hydroxydopamine * MPTP * Rotenone Rotenone is an odorless, colorless, crystalline isoflavone. It occurs naturally in the seeds and stems of several plants, such as the jicama vine, and in the roots of several other members of the Fabaceae. It was the first-described member of the ... References {{Phenethylamines Catechols Human pathological m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |