|
ACTN2
Alpha-actinin-2 is a protein which in humans is encoded by the ''ACTN2'' gene. This gene encodes an alpha-actinin isoform that is expressed in both skeletal and cardiac muscles and functions to anchor myofibrillar actin thin filaments and titin to Z-discs. Structure Alpha-actinin-2 is a 103.8 kDa protein composed of 894 amino acids. Each molecule is rod-shaped (35 nm in length) and it homodimerizes in an anti-parallel fashion. Each monomer has an N-terminal actin-binding region composed of two calponin homology domains, two C-terminal EF hand domains, and four tandem spectrin-like repeats form the rod domain in the central region of the molecule. The high-resolution crystal structure of human alpha-actinin 2 at 3.5 Å was recently resolved. Alpha actinins belong to the spectrin gene superfamily which represents a diverse group of actin-binding cytoskeletal proteins, including spectrin, dystrophin, utrophin and fimbrin. Skeletal, cardiac, and smooth muscle isoforms are local ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MYOT
Myotilin is a protein that in humans is encoded by the ''MYOT'' gene. Myotilin (myofibrillar titin-like protein) also known as TTID (TiTin Immunoglobulin Domain) is a muscle protein that is found within the Z-disc of sarcomeres. Structure Myotilin is a 55.3 kDa protein composed of 496 amino acids. Myotilin was originally identified as a novel alpha-actinin binding partner with two Ig-like domains, that localized to the Z-disc. The I-type Ig-like domains reside at the C-terminal half, and are most homologous to Ig domains 2-3 of palladin and Ig domains 4-5 of myopalladin and more distantly related to Z-disc Ig domains 7 and 8 of titin. The C-terminal region hosts the binding sites for Z-band proteins, and 2 Ig domains are the site of homodimerization for myotilin. By contrast, the N-terminal part of myotilin is unique, consisting of a serine-rich region with no homology to known proteins. Several disease-associated mutations involve serine residues within the serine-rich d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinin
Actinin is a microfilament protein. The functional protein is an anti-parallel dimer, which cross-links the thin filaments in adjacent sarcomeres, and therefore coordinates contractions between sarcomeres in the horizontal axis. Alpha-actinin is a part of the spectrin superfamily. This superfamily is made of spectrin, dystrophin, and their homologous and isoforms. In non-muscle cells, it is found by the actin filaments and at the adhesion sites. The lattice like arrangement provides stability to the muscle contractile apparatus. Specifically, it helps bind actin filaments to the cell membrane. There is a binding site at each end of the rod and with bundles of actin filaments. The non-sarcomeric alpha-actinins, encoded by '' ACTN1'' and '' ACTN4'', are widely expressed. '' ACTN2'' expression is found in both cardiac and skeletal muscle, whereas ''ACTN3'' is limited to the latter. Both ends of the rod-shaped alpha-actinin dimer contain actin-binding domains. Six different protei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MYPN
Myopalladin is a protein that in humans is encoded by the ''MYPN'' gene. Myopalladin is a muscle protein responsible for tethering proteins at the Z-disc and for communicating between the sarcomere and the nucleus in cardiac and skeletal muscle Structure Myopalladin is a 145.2 kDa protein composed of 1320 amino acids. Myopalladin has five Ig-like repeats within the protein, and a proline-rich domain. Myopalladin binds the Src homology domain of nebulette and nebulin and tethers it to alpha-actinin via its C-terminal domain binding to the EF hand domains of alpha-actinin. The N-terminal region of myopalladin binds to the nuclear protein CARP, known to regulate gene expression in muscle. It also has been shown to bind ANKRD23. Function Myopalladin has dual subcellular localization, residing in both the nucleus and sarcomere/ I-bands in muscle. Accordingly, myopalladin has functions in both sarcomere assembly and in control of gene expression. Specifics of these functions w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DISC1
Disrupted in schizophrenia 1 is a protein that in humans is encoded by the ''DISC1'' gene. In coordination with a wide array of interacting partners, DISC1 has been shown to participate in the regulation of cell proliferation, differentiation, migration, neuronal axon and dendrite outgrowth, mitochondrial transport, fission and/or fusion, and cell-to-cell adhesion. Several studies have shown that unregulated expression or altered protein structure of DISC1 may predispose individuals to the development of schizophrenia, clinical depression, bipolar disorder, and other psychiatric conditions. The cellular functions that are disrupted by permutations in DISC1, which lead to the development of these disorders, have yet to be clearly defined and are the subject of current ongoing research. Although recent genetic studies of large schizophrenia cohorts have failed to implicate DISC1 as a risk gene at the gene level, the DISC1 interactome gene set was associated with schizophrenia, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SYNPO2
Myopodin protein, also called Synaptopodin-2 is a protein that in humans is encoded by the ''SYNPO2'' gene. Myopodin is expressed in cardiac, smooth muscle and skeletal muscle, and localizes to Z-disc structures. Structure Myopodin is a 117.4 kDa protein composed of 1093 amino acids, although four alternatively-spliced isoforms have been described. Myopodin contains one PPXY motif, multiple PXXP motifs, and two potential nuclear localization sequences (one N-terminal and one C-terminal). PPXY motifs have been shown to mediate interactions, and PXXP motifs represent potential sites of interaction for SH3 domain-containing proteins. Myopodin contains a novel actin binding site (between amino acids 410 and 563) in the center of the protein. Function During myotube differentiation, myopodin interacts with stress fibers prior to co-localizing with alpha actinin-2 at Z-discs in mature striated muscle cells. Myopodin has been shown to shuttle between the nucleus and cytoplasm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EF Hand
The EF hand is a helix–loop–helix structural domain or ''motif'' found in a large family of calcium-binding proteins. The EF-hand motif contains a helix–loop–helix topology, much like the spread thumb and forefinger of the human hand, in which the Ca2+ ions are coordinated by ligands within the loop. The motif takes its name from traditional nomenclature used in describing the protein parvalbumin, which contains three such motifs and is probably involved in muscle relaxation via its calcium-binding activity. The EF-hand consists of two alpha helices linked by a short loop region (usually about 12 amino acids) that usually binds calcium ions. EF-hands also appear in each structural domain of the signaling protein calmodulin and in the muscle protein troponin-C. Calcium ion binding site The calcium ion is coordinated in a pentagonal bipyramidal configuration. The six residues involved in the binding are in positions 1, 3, 5, 7, 9 and 12; these residues are denoted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ADAM12
Disintegrin and metalloproteinase domain-containing protein 12 (previously Meltrin) is an enzyme that in humans is encoded by the ''ADAM12'' gene. ADAM12 has two splice variants: ADAM12-L, the long form, has a transmembrane region and ADAM12-S, a shorter variant, is soluble and lacks the transmembrane and cytoplasmic domains. Function This gene encodes a member of the ADAM (a disintegrin and metalloprotease) protein family. Members of this family are membrane-anchored proteins structurally related to snake venom disintegrins, and have been implicated in a variety of biological processes involving cell-cell and cell-matrix interactions, including fertilization, muscle development, and neurogenesis. This gene has two alternatively spliced transcripts: a shorter secreted form and a longer membrane-bound form. The shorter form is found to stimulate myogenesis. Clinical Significance ADAM 12, a metalloprotease that binds insulin growth factor binding protein-3 (IGFBP-3), appear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LDB3
LIM domain binding 3 (LDB3), also known as Z-band alternatively spliced PDZ-motif (ZASP), is a protein which in humans is encoded by the ''LDB3'' gene. ZASP belongs to the Enigma subfamily of proteins and stabilizes the sarcomere (the basic units of muscles) during contraction, through interactions with actin in cardiac and skeletal muscles. Mutations in the ZASP gene has been associated with several muscular diseases. Structure ZASP is a PDZ domain-containing protein. PDZ motifs are modular protein-protein interaction domains consisting of 80-120 amino acid residues. PDZ domain-containing proteins interact with each other in cytoskeletal assembly or with other proteins involved in targeting and clustering of membrane proteins. ZASP interacts with alpha-actinin-2 through its N-terminal PDZ domain and with protein kinase C via its C-terminal LIM domains. The LIM domain is a cysteine-rich motif defined by 50-60 amino acids containing two zinc-binding modules. This protein also in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMDAR
The ''N''-methyl-D-aspartate receptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and predominantly Ca2+ ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a "coincidence detector" and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions. The NMDA receptor is ionotropic, meaning it is a protein which allows the passage of ions through ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
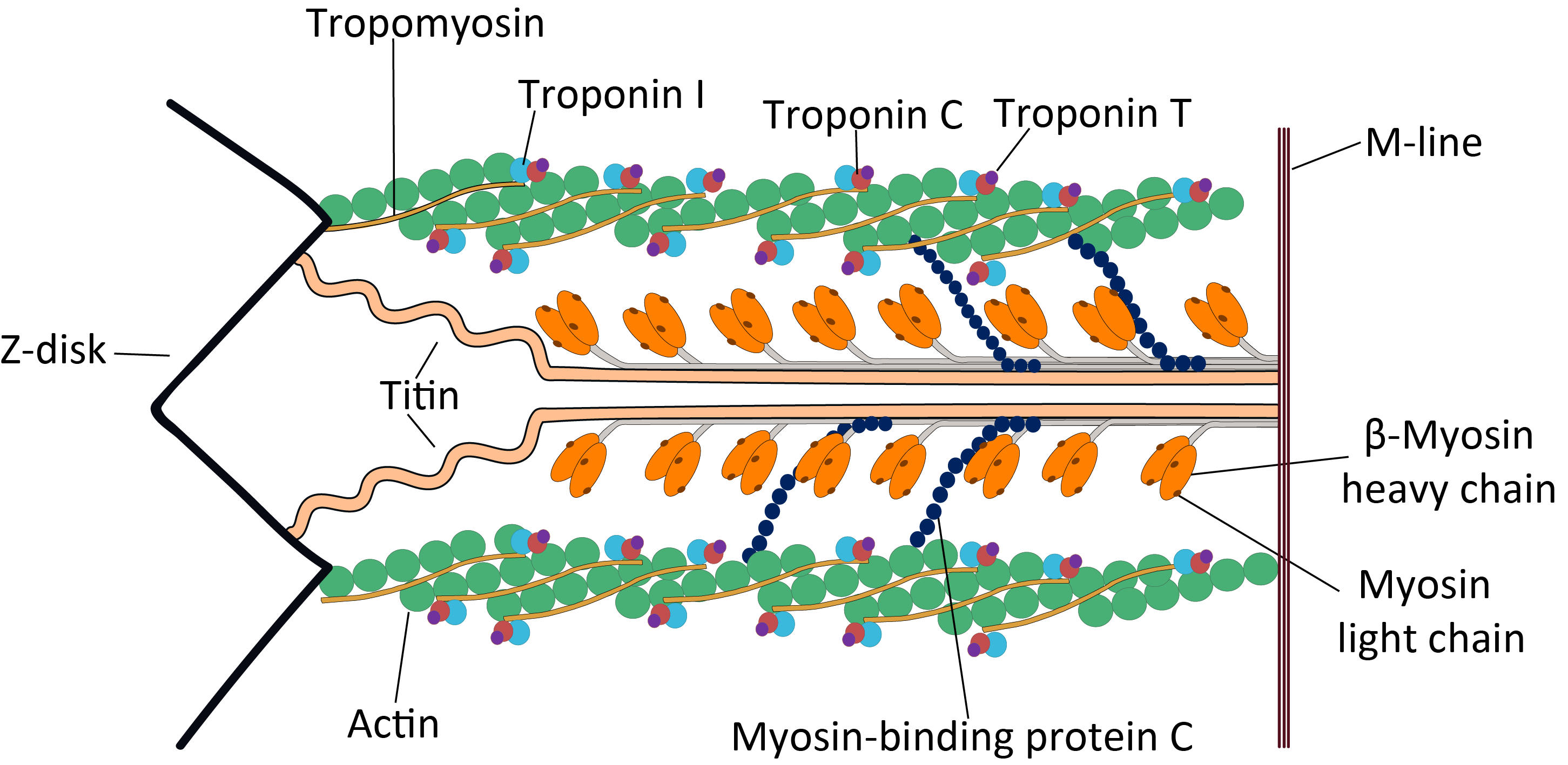
Titin
Titin (; also called connectin) is a protein that in humans is encoded by the ''TTN'' gene. The protein, which is over 1 μm in length, functions as a molecular spring that is responsible for the passive elasticity of muscle. It comprises 244 individually folded protein domains connected by unstructured peptide sequences. These domains unfold when the protein is stretched and refold when the tension is removed. Titin is important in the contraction of striated muscle tissues. It connects the Z disc to the M line in the sarcomere. The protein contributes to force transmission at the Z disc and resting tension in the I band region. It limits the range of motion of the sarcomere in tension, thus contributing to the passive stiffness of muscle. Variations in the sequence of titin between different types of striated muscle ( cardiac or skeletal) have been correlated with differences in the mechanical properties of these muscles. Titin is the third most abundant protein in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them ( phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein ( substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase. The great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets. Most of the others are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction. Chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |