|
ACTG1
Actin, cytoplasmic 2, or gamma-actin is a protein that in humans is encoded by the ''ACTG1'' gene. Gamma-actin is widely expressed in cellular cytoskeletons of many tissues; in adult striated muscle cells, gamma-actin is localized to Z-discs and costamere structures, which are responsible for force transduction and transmission in muscle cells. Mutations in ''ACTG1'' have been associated with nonsyndromic hearing loss and Baraitser-Winter syndrome, as well as susceptibility of adolescent patients to vincristine toxicity. Structure Human gamma-actin is 41.8 kDa in molecular weight and 375 amino acids in length. Actins are highly conserved proteins that are involved in various types of cell motility, and maintenance of the cytoskeleton. In vertebrates, three main groups of actin paralogs, alpha, beta, and gamma, have been identified. The alpha actins are found in muscle tissues and are a major constituent of the sarcomere contractile apparatus. The beta and gamma actins co- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-actin
Actin is a protein family, family of Globular protein, globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in myofibril, muscle fibrils. It is found in essentially all Eukaryote, eukaryotic cells, where it may be present at a concentration of over 100 micromolar, μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm. An actin protein is the monomeric Protein subunit, subunit of two types of filaments in cells: microfilaments, one of the three major components of the cytoskeleton, and thin filaments, part of the Muscle contraction, contractile apparatus in muscle cells. It can be present as either a free monomer called G-actin (globular) or as part of a linear polymer microfilament called F-actin (filamentous), both of which are essential for such important cellular functions as the Motility, mobility and contraction of cell (biology), cells during cell division. Actin participates in many important cellular pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsyndromic Deafness
Nonsyndromic deafness is hearing loss that is not associated with other signs and symptoms. In contrast, syndromic deafness involves hearing loss that occurs with abnormalities in other parts of the body. Nonsyndromic deafness constitutes 75% of all hearing loss cases, and an estimated 100 genes are thought to be linked to this condition. About 80% are linked to autosomal recessive inheritance, 15% to autosomal dominant inheritance, 1-3% through the X chromosome, and 0.5-1% are associated with mitochondrial inheritance. Genetic changes are related to the following types of nonsyndromic deafness: * DFNA: nonsyndromic deafness, autosomal dominant * DFNB: nonsyndromic deafness, autosomal recessive * DFNX: nonsyndromic deafness, X-linked * nonsyndromic deafness, mitochondrial Each type is numbered in the order in which it was described. For example, DFNA1 was the first described autosomal dominant type of nonsyndromic deafness. Mitochondrial nonsyndromic deafness involves changes to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dystrophin
Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costamere or the dystrophin-associated protein complex (DAPC). Many muscle proteins, such as α-dystrobrevin, syncoilin, synemin, sarcoglycan, dystroglycan, and sarcospan, colocalize with dystrophin at the costamere. It has a molecular weight of 427 kDa. Dystrophin is coded for by the ''DMD'' gene – the largest known human gene, covering 2.4 megabases (0.08% of the human genome) at Locus (genetics), locus X chromosome, Xp21. The primary transcript in muscle measures about 2,100 kilobases and takes 16 hours to transcribe; the Mature messenger RNA, mature mRNA measures 14.0 kilobases. The 79-exon muscle transcript codes for a protein of 3685 amino acid residues. Spontaneous or inherited mutations in the dystrophin gene can cause different form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the proteinogenic amino acids. Only the L- stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, '' sericum''. Serine's structure was established in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other non-essential amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. Methionine is also involved in angiogenesis and various processes related to DNA transcription, epigenetic expression, and gene regulation. Methionine was first isolated in 1921 by John Howard Mueller. It is Genetic code, encoded by the codon AUG. It was named by Satoru Odake in 1925, as an abbreviation of its structural description 2-amino-4-(methylthio)butanoic acid. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the proton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it. Essential amino acids are necessary in the human diet. In plants isoleucine can be synthesized from threonine and methionine. In plants and bacteria, isoleucine is synthesized from a pyruvate employing leucine biosynthesis enzymes. It is encoded by the codons AUU, AUC, and AUA. Metabolism Biosynthesis In plants and microorganisms, isoleucine is synthesized from pyruvate and alpha-ketobutyrate. This pathway ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− form when dissolved in water), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myopathy
In medicine, myopathy is a disease of the muscle in which the muscle fibers do not function properly. ''Myopathy'' means muscle disease ( Greek : myo- ''muscle'' + patheia '' -pathy'' : ''suffering''). This meaning implies that the primary defect is within the muscle, as opposed to the nerves (" neuropathies" or " neurogenic" disorders) or elsewhere (e.g., the brain). This muscular defect typically results in myalgia (muscle pain), muscle weakness (reduced muscle force), or premature muscle fatigue (initially normal, but declining muscle force). Muscle cramps, stiffness, spasm, and contracture can also be associated with myopathy. Myopathy experienced over a long period (chronic) may result in the muscle becoming an abnormal size, such as muscle atrophy (abnormally small) or a pseudoathletic appearance (abnormally large). Capture myopathy can occur in wild or captive animals, such as deer and kangaroos, and leads to morbidity and mortality. It usually occurs as a result o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myocytes
A muscle cell, also known as a myocyte, is a mature contractile cell in the muscle of an animal. In humans and other vertebrates there are three types: skeletal, smooth, and cardiac (cardiomyocytes). A skeletal muscle cell is long and threadlike with many nuclei and is called a ''muscle fiber''. Muscle cells develop from embryonic precursor cells called myoblasts. Skeletal muscle cells form by fusion of myoblasts to produce multinucleated cells (syncytia) in a process known as myogenesis. Skeletal muscle cells and cardiac muscle cells both contain myofibrils and sarcomeres and form a striated muscle tissue. Cardiac muscle cells form the cardiac muscle in the walls of the heart chambers, and have a single central nucleus. Cardiac muscle cells are joined to neighboring cells by intercalated discs, and when joined in a visible unit they are described as a ''cardiac muscle fiber''. Smooth muscle cells control involuntary movements such as the peristalsis contractions in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
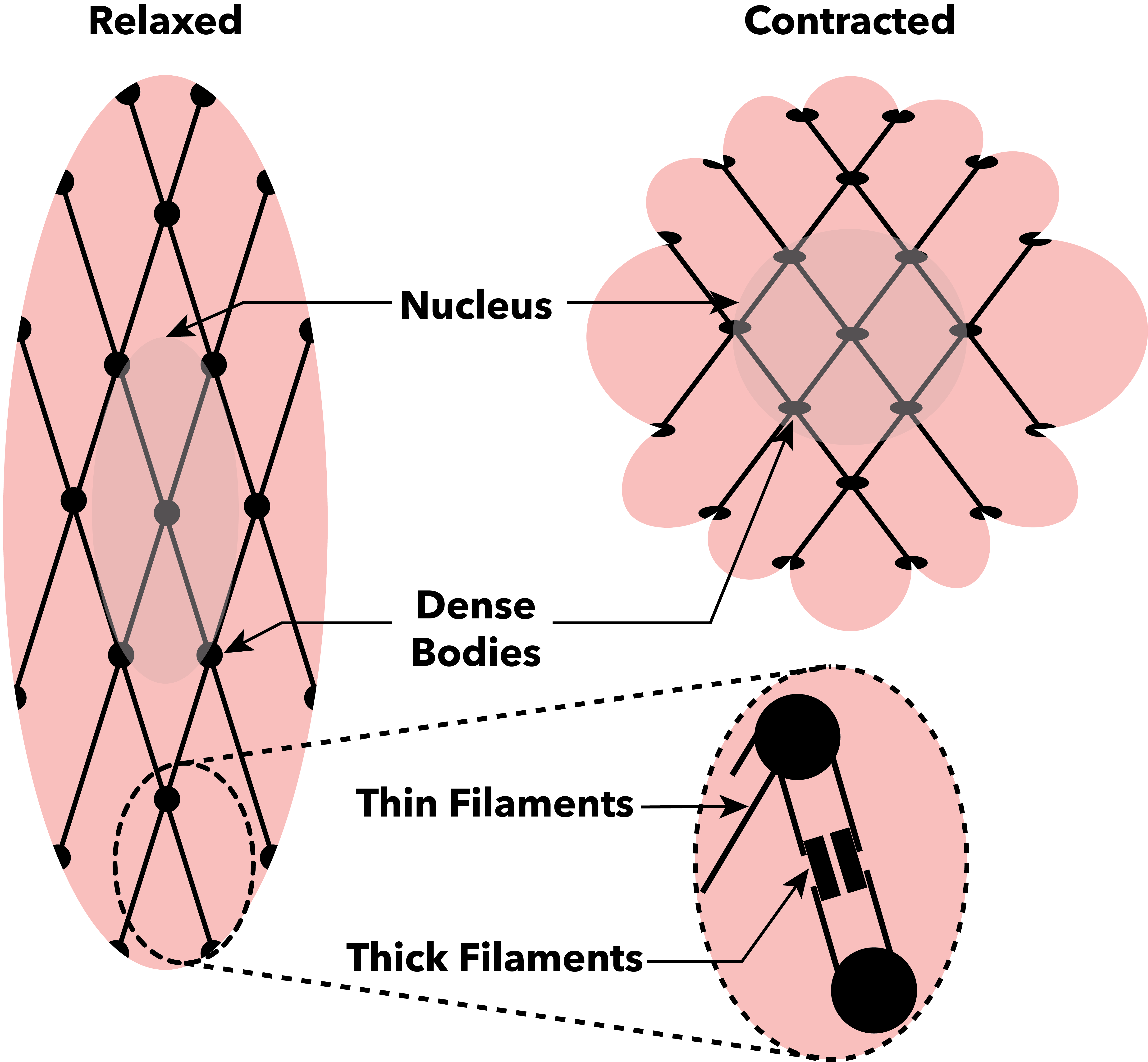
Muscle Contraction
Muscle contraction is the activation of Tension (physics), tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state. For the contractions to happen, the muscle cells must rely on the change in action of two types of Myofilament, filaments: thin and thick filaments. The major constituent of thin filaments is a chain formed by helical coiling of two strands of actin, and thick filaments dominantly consist of chains of the Motor protein, motor-protein myosin. Together, these two filaments form myofibrils - the basic functional organelles in the skeletal muscle system. In vertebrates, Muscle cell#Muscle contraction in striated muscle, skele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Necrosis
Necrosis () is a form of cell injury which results in the premature death of cells in living tissue by autolysis. The term "necrosis" came about in the mid-19th century and is commonly attributed to German pathologist Rudolf Virchow, who is often regarded as one of the founders of modern pathology. Necrosis is caused by factors external to the cell or tissue, such as infection, or trauma which result in the unregulated digestion of cell components. In contrast, ''apoptosis'' is a naturally occurring programmed and targeted cause of cellular death. While apoptosis often provides beneficial effects to the organism, necrosis is almost always detrimental and can be fatal. Cellular death due to necrosis does not follow the apoptotic signal transduction pathway, but rather various receptors are activated and result in the loss of cell membrane integrity and an uncontrolled release of products of cell death into the extracellular space. This initiates an inflammatory response in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |