|
5-Methyltryptamine
5-Methyltryptamine (5-MeT, 5-Me-T) is a non-selective serotonin receptor agonist and serotonin releasing agent of the tryptamine family that has been used in scientific research. It is related to other 5-substituted tryptamines such as serotonin (5-hydroxytryptamine; 5-HT) and 5-methoxytryptamine (5-MeO-T). The compound is also a positional isomer of ''N''-methyltryptamine (NMT). Pharmacology 5-MeT is known to act as a potent serotonin 5-HT2A receptor full agonist, with an of 6.00nM and an of 100%. In addition, it is known to be a ligand of the serotonin 5-HT1A and 5-HT2B receptors and an agonist of the serotonin 5-HT1D and 5-HT2C receptors. Similarly to tryptamine and 5-MeO-T, but in contrast to serotonin, 5-MeT shows very low potency as an agonist of the serotonin 5-HT3 receptor ( = 60,000nM). In addition to acting as an agonist of various serotonin receptors, 5-MeT is a monoamine releasing agent (MRA), with high selectivity for induction of serotonin release over i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Releasing Agent
A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of one or more monoamine neurotransmitters from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitters and hence enhanced signaling by those neurotransmitters. The monoamine neurotransmitters include serotonin, norepinephrine, and dopamine; MRAs can induce the release of one or more of these neurotransmitters. MRAs work by reversing the direction of the monoamine transporters (MATs), including the serotonin transporter (SERT), norepinephrine transporter (NET), and/or dopamine transporter (DAT), causing them to promote efflux of non-vesicular cytoplasmic monoamine neurotransmitter rather than reuptake of synaptic monoamine neurotransmitter. Many, but not all MRAs, also reverse the direction of the vesicular monoamine transporter 2 (VMAT2), thereby additionally resulting in efflux of vesicular monoamine neuro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Receptor Agonist
A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin (5-hydroxytryptamine; 5-HT), a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors. Non-selective agonists Serotonergic psychedelics such as tryptamines (e.g., psilocybin, psilocin, , 5-MeO-DMT, bufotenin), lysergamides (e.g., , ergine ()), phenethylamines (e.g., mescaline, 2C-B, 25I-NBOMe), and amphetamines (e.g., , ) are non-selective agonists of serotonin receptors. Their hallucinogenic effects are specifically mediated by activation of the 5-HT2A receptor. Drugs that increase extracellular serotonin levels such as serotonin reuptake inhibitors (e.g., fluoxetine, venlafaxine), serotonin releasing agents (e.g., fenfluramine, ), and monoamine oxidase inhibitors (e.g., phenelzine, moclobemide) are indirect non-selective serotonin receptor agonists. They are used variously as antidepressant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
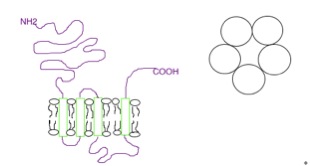
5-HT1A Receptor
The serotonin 1A receptor (or 5-HT1A receptor) is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene. Distribution The 5-HT1A receptor is the most widespread of all the 5-HT receptors. In the central nervous system, 5-HT1A receptors exist in the cerebral cortex, hippocampus, Septum pellucidum, septum, amygdala, and Raphe nuclei, raphe nucleus in high densities, while low amounts also exist in the basal ganglia and thalamus. The 5-HT1A receptors in the raphe nucleus are largely somatodendritic autoreceptors, whereas those in other areas such as the hippocampus are postsynaptic receptors. Function Neur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule. Numeric locants The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of numeric prefixes to indicate the position of substituents, generally by identifying the parent hydrocarbon chain and assigning the carbon atoms based on their substituents in order of precedence. For example, there are at least two isomers of the linear form of pentanone, a ketone that contains a chain of exactly five carbon atoms. There is an oxygen atom bonded to one of the middle three carbons (if it were bonded to an end carbon, the molecule would be an aldehyde, not a ketone), but it is not clear where it is located. In this example, the carbon atoms are numbered from one to five, which starts at one end and proceeds sequentially along the chain. Now the position of the oxygen atom can be defined as on carbon atom number two, three o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synaptosome
A synaptosome is an isolated synaptic terminal from a neuron. Synaptosomes are obtained by mild homogenization of nervous tissue under isotonic conditions and subsequent fractionation using differential and density gradient centrifugation. Liquid shear detaches the nerve terminals from the axon and the plasma membrane surrounding the nerve terminal particle reseals. Synaptosomes are osmotically sensitive, contain numerous small clear synaptic vesicles, sometimes larger dense-core vesicles and frequently one or more small mitochondria. They carry the morphological features and most of the chemical properties of the original nerve terminal. Synaptosomes isolated from mammalian brain often retain a piece of the attached postsynaptic membrane, facing the active zone. Synaptosomes were first isolated in an attempt to identify the subcellular compartment corresponding to the fraction of so-called bound acetylcholine that remains when brain tissue is homogenized in iso-osmotic sucrose. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norepinephrine
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic compound, organic chemical in the catecholamine family that functions in the brain and human body, body as a hormone, neurotransmitter and neuromodulator. The name "noradrenaline" (from Latin '':wikt:ad-, ad'', "near", and '':wikt:ren, ren'', "kidney") is more commonly used in the United Kingdom and the rest of the world, whereas "norepinephrine" (from Ancient Greek :wikt:ἐπί, ἐπῐ́ (''epí''), "upon", and :wikt:νεφρός, νεφρός (''nephrós''), "kidney") is usually preferred in the United States. "Norepinephrine" is also the international nonproprietary name given to norepinephrine (drug), the drug. Regardless of which name is used for the substance itself, parts of the body that produce or are affected by it are referred to as noradrenergic. The general function of norepinephrine is to mobilize the brain and body for action. Norepinephrine release is lowest during sleep, rise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and many addictive drugs increase dopamine release or block its reuptake into neurons following release. Other brain dopamine pathways are involved in motor control and in controllin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Receptor
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in multiple tissues including the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin (i.e., 5-hydroxytryptamine, hence "5-HT") receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand. The serotonin receptors modulate the release of many neurotransmitters, including glutamate, GABA, dopamine, epinephrine / norepinephrine, and acetylcholine, as well as many hormones, including oxytocin, prolactin, vasopressin, cortisol, corticotropin, and substance P, among others. Serotonin receptors influence various biological and neurological processes such as aggression, anxiety, appetite, cognition, learning, memory, mood, nausea, sleep, and thermoregulation. They are the target of a variety of pharmaceutical and recreational drugs, inclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT3 Receptor
The 5-HT3 receptor belongs to the Cys-loop superfamily of ligand-gated ion channels (LGICs) and therefore differs structurally and functionally from all other 5-HT receptors (5-hydroxytryptamine, or serotonin receptors) which are G-protein-coupled receptor, G protein-coupled receptors. This ion channel is cation-selective and mediates neuronal depolarization and excitation within the Central nervous system, central and Peripheral nervous system, peripheral nervous systems. As with other ligand gated ion channels, the 5-HT3 receptor consists of five subunits arranged around a central ion conducting pore, which is permeable to sodium (Na), potassium (K), and calcium (Ca) ions. Binding of the neurotransmitter 5-hydroxytryptamine (serotonin) to the 5-HT3 receptor opens the channel, which, in turn, leads to an excitatory response in neurons. The rapidly activating, desensitizing, inward current is predominantly carried by sodium and potassium ions. 5-HT3 receptors have a negligible perme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid tryptophan. The chemical structure is defined by an indole—a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate serotonin receptors and trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are bei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2C Receptor
The 5-HT2C receptor is a subtype of the 5-HT2 receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, it is a G protein-coupled receptor (GPCR) that is coupled to Gq/G11 and mediates excitatory neurotransmission. ''HTR2C'' denotes the human gene encoding for the receptor, that in humans is located on the X chromosome. As males have one copy of the gene and females have one of the two copies of the gene repressed, polymorphisms at this receptor can affect the two sexes to differing extent. Structure At the cell surface the receptor exists as a homodimer. The crystal structure has been known since 2018. Distribution 5-HT2C receptors are located mainly in the choroid plexus, and in rats is also found in many other brain regions in high concentrations, including parts of the hippocampus, anterior olfactory nucleus, substantia nigra, several brainstem nuclei, amygdala, subthalamic nucleus and lateral habenula. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |