|
5-Methoxytryptamines
5-Methoxytryptamine (5-MT, 5-MeO-T, or 5-OMe-T), also known as serotonin methyl ether or ''O''-methylserotonin and as mexamine, is a tryptamine derivative closely related to the neurotransmitters serotonin and melatonin. It has been shown to occur naturally in the body in low levels, especially in the pineal gland. It is formed via ''O''-methylation of serotonin or ''N''-deacetylation of melatonin. 5-MT is a highly potent and non-selective serotonin receptor agonist and shows serotonergic psychedelic-like effects in animals. However, it is inactive in humans, at least orally, likely due to rapid metabolism by monoamine oxidase (MAO). The levels and effects of 5-MT are dramatically potentiated by monoamine oxidase inhibitors (MAOIs) in animals. Biosynthesis 5-MT can be formed by ''O''-methylation of serotonin mediated by hydroxyindole ''O''-methyltransferase (HIOMT) or by ''N''-deacetylation of melatonin. It is also a precursor of 5-MeO-DMT in some species. Pharmacology Ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melatonin
Melatonin, an indoleamine, is a natural compound produced by various organisms, including bacteria and eukaryotes. Its discovery in 1958 by Aaron B. Lerner and colleagues stemmed from the isolation of a substance from the pineal gland of cows that could induce skin lightening in common frogs. This compound was later identified as a hormone secreted in the brain during the night, playing a crucial role in regulating the sleep-wake cycle, also known as the circadian rhythm, in vertebrates. In vertebrates, melatonin's functions extend to Entrainment (chronobiology), synchronizing sleep-wake cycles, encompassing sleep-wake timing and blood pressure regulation, as well as controlling seasonal rhythmicity (circannual cycle), which includes reproduction, fattening, molting, and hibernation. Its effects are mediated through the activation of melatonin receptors and its role as an antioxidant. In plants and bacteria, melatonin primarily serves as a defense mechanism against oxidative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-MeO-DMT
5-MeO-DMT (5-methoxy-''N'',''N''-dimethyltryptamine), also known as ''O''-methylbufotenin or mebufotenin (), is a naturally occurring psychedelic of the tryptamine family. It is found in a wide variety of plant species, and is also secreted by the glands of at least one toad species, the Colorado River toad. It may occur naturally in humans as well. Like its close relatives dimethyltryptamine (DMT) and bufotenin (5-HO-DMT), it has been used as an entheogen in South America. Slang terms include ''five-methoxy'', ''the power'', ''bufo'', and ''toad venom''. The drug has been described as the most powerful psychedelic and, by journalist Michael Pollan, as the "Mount Everest of psychedelics". Adverse effects of 5-MeO-DMT include sickness, vomiting, headache, chest pressure, fatigue, anxiety, fear, terror, confusion, paranoia, crying, loss of awareness and motor control, and reactivations. The drug acts as a non-selective serotonin receptor agonist, including of the sero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration whereby a substance is taken through the Human mouth, mouth, swallowed, and then processed via the digestive system. This is a common route of administration for many medications. Oral administration can be easier and less painful than other routes of administration, such as Injection (medicine), injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth". The expression is used in medicine to describe a treatment that is taken orally (but not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
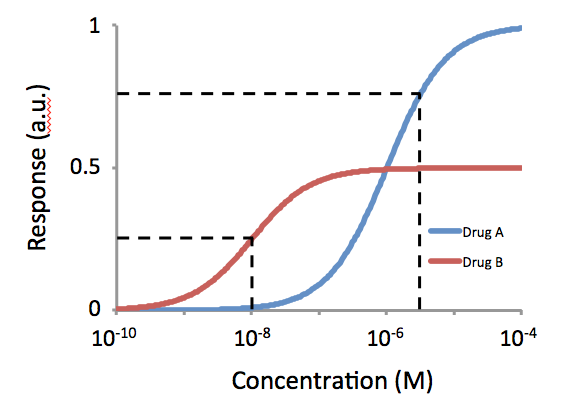
Potency (pharmacology)
In pharmacology, potency or biological potency is a measure of a drug's biological activity expressed in terms of the dose required to produce a pharmacological effect of given intensity. A highly potent drug (e.g., fentanyl, clonazepam, risperidone, benperidol, bumetanide) evokes a given response at low concentrations, while a drug of lower potency (e.g. morphine, alprazolam, ziprasidone, haloperidol, furosemide) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness nor more side effects nor less side effects. Types of potency The International Union of Basic and Clinical Pharmacology (IUPHAR) has stated that "potency is an imprecise term that should always be further defined", and lists of types of potency as follows: Miscellaneous Lysergic acid diethylamide (LSD) is one of the most potent psychoactive drug A psychoactive drug, psychopharmaceutical, mind-altering drug, consciousness-altering drug, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT1B Receptor
5-hydroxytryptamine receptor 1B also known as the 5-HT1B receptor is a protein that in humans is encoded by the ''HTR1B'' gene. The 5-HT1B receptor is a 5-HT receptor subtype. Tissue distribution and function 5-HT1B receptors are widely distributed throughout the central nervous system with the highest concentrations found in the frontal cortex, basal ganglia, striatum, and the hippocampus. The function of the 5-HT1B receptor differs depending upon its location. In the frontal cortex, it is believed to act as a terminal receptor inhibiting the release of dopamine. In the basal ganglia and the striatum, evidence suggests 5-HT signaling acts on an autoreceptor, inhibiting the release of serotonin and decreasing glutamatergic transmission by reducing miniature excitatory postsynaptic potential (mEPSP) frequency, respectively. In the hippocampus, a recent study has demonstrated that activation of postsynaptic 5-HT1B heteroreceptors produces a facilitation in excitatory synapt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT1A Receptor
The serotonin 1A receptor (or 5-HT1A receptor) is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene. Distribution The 5-HT1A receptor is the most widespread of all the 5-HT receptors. In the central nervous system, 5-HT1A receptors exist in the cerebral cortex, hippocampus, Septum pellucidum, septum, amygdala, and Raphe nuclei, raphe nucleus in high densities, while low amounts also exist in the basal ganglia and thalamus. The 5-HT1A receptors in the raphe nucleus are largely somatodendritic autoreceptors, whereas those in other areas such as the hippocampus are postsynaptic receptors. Function Neur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affinity (pharmacology)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biological Target
A biological target is anything within a living organism to which some other entity (like an endogenous ligand or a drug) is directed and/or binds, resulting in a change in its behavior or function. Examples of common classes of biological targets are proteins and nucleic acids. The definition is context-dependent, and can refer to the biological target of a pharmacologically active drug compound, the receptor target of a hormone (like insulin), or some other target of an external stimulus. Biological targets are most commonly proteins such as enzymes, ion channels, and receptors. Mechanism The external stimulus (''i.e.'', the drug or ligand) physically binds to ("hits") the biological target. The interaction between the substance and the target may be: * noncovalent – A relatively weak interaction between the stimulus and the target where no chemical bond is formed between the two interacting partners and hence the interaction is completely reversible. * reversible c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precursor (biochemistry)
In chemistry, a precursor is a compound that participates in a chemical reaction that produces another compound. In biochemistry, the term "precursor" often refers more specifically to a chemical compound preceding another in a metabolic pathway, such as a protein precursor. Illicit drug precursors In 1988, the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances introduced detailed provisions and requirements relating the control of precursors used to produce drugs of abuse. In Europe the Regulation (EC) No. 273/2004 of the European Parliament and of the Council on drug precursors was adopted on 11 February 2004. (European law on drug precursors) Illicit explosives precursors On January 15, 2013, the Regulation (EU) No. 98/2013 of the European Parliament and of the Council on the marketing and use of explosives precursors was adopted. The Regulation harmonises rules across Europe on the making available, introduction, possession and u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylserotonin O-methyltransferase
N-Acetylserotonin O-methyltransferase, also known as ASMT, is an enzyme which catalyzes the final reaction in melatonin biosynthesis: converting Normelatonin to melatonin. This reaction is embedded in the more general tryptophan metabolism pathway. The enzyme also catalyzes a second reaction in tryptophan metabolism: the conversion of 5-hydroxy-indoleacetate to 5-methoxy-indoleacetate. The other enzyme which catalyzes this reaction is n-acetylserotonin-o-methyltransferase-like-protein. In humans the ASMT enzyme is encoded by the pseudoautosomal ''ASMT'' gene. A copy exists near the endcaps of the short arms of both the X chromosome and the Y chromosome. Structure and gene location ''N-Acetylserotonin O-methyltransferase'' is an enzyme that is coded for by genes located on the pseudoautosomal region of the X and Y chromosome, and is most abundantly found in the pineal gland and retina of humans. The structure of ''N- Acetylserotonin O-methyltransferase'' has been determined ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
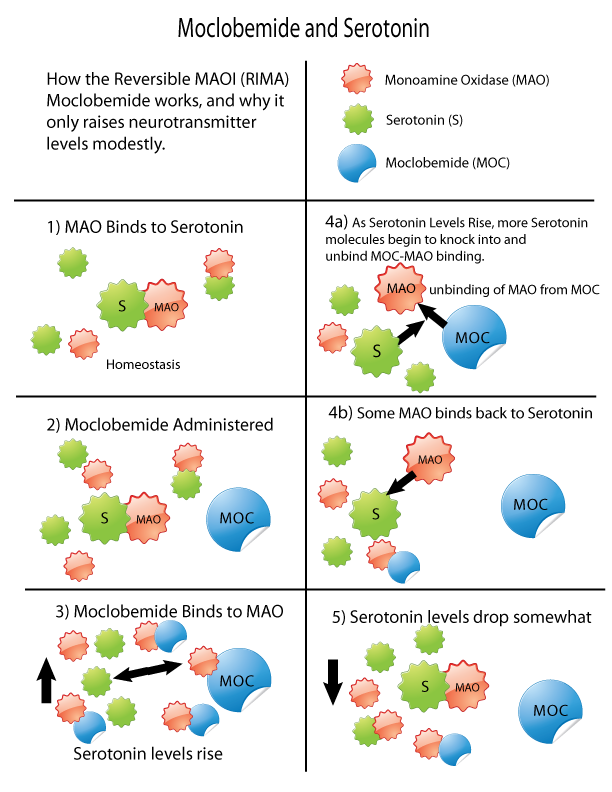
Monoamine Oxidase Inhibitor
Monoamine oxidase inhibitors (MAOIs) are a drug class, class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that binding selectivity, selectively and Enzyme inhibitor#Reversible inhibitors, reversibly enzyme inhibitor, inhibit the MAO-A enzyme. RIMAs are used clinically in the medication, treatment of major depressive disorder, depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase
Monoamine oxidases (MAO) () are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases. MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate monoamine neurotransmitters. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with monoamine oxidase inhibitors (MAOIs) which block the action of MAOs. Subtypes and tissue distribution In humans there are two types of MAO: MAO-A and MAO-B. * Both are found in neurons and astroglia. * Outside the central nervous system: ** MAO-A is also found in the liver, pulmonary vascular end ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |