|
3-Ethyl-3-pentanol
3-Ethyl-3-pentanol, also known as 3-ethylpentan-3-ol, is a tertiary alcohol with the molecular formula C7H16O. It reacts with chromic acid by first dehydrating to an olefin 3-ethyl-2-pentene, and then by converting the double bond to an epoxide. Perfluorination affords perfluorotriethylcarbinol, a powerful uncoupling agent An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transp .... References Alkanols Tertiary alcohols Ethyl compounds {{alcohol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorotriethylcarbinol
Perfluorotriethylcarbinol is a perfluorinated alcohol, namely 3-ethyl-3-pentanol. It is a powerful uncoupling agent and is toxic by inhalation. See also *Perfluorinated compound *Uncoupling agent An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transp ... References Uncouplers Perfluorinated alcohols Tertiary alcohols {{Alcohol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384â322 BCE), Theophrastus (â287 BCE), and Pliny the Elder (23/24â79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, JÄ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
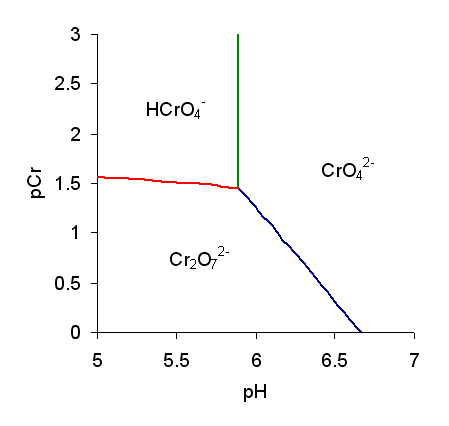
Chromic Acid
Chromic acid is a chemical compound with the chemical formula . It is also a jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide. The term "chromic acid" is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the molecular species, of which the trioxide is the anhydride. Chromic acid features chromium in an oxidation state of +6 (and a valence of VI or 6). It is a strong and corrosive oxidizing agent and a moderate carcinogen. Molecular chromic acid Molecular chromic acid, , in principle, resembles sulfuric acid, . It would ionize accordingly: : The p''K''a for the equilibrium is not well characterized. Reported values vary between about â0.8 to 1.6. The structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is called an ''epoxy''. However, few if any of the epoxy groups i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorinated Compound
A perfluorinated compound (PFC) or perfluoro compound is an Organofluorine chemistry, organofluorine compound that lacks C-H bonds. Many perfluorinated compounds have properties that are quite different from their C-H containing analogues. Common functional groups in PFCs are Alcohol (chemistry), OH, Perfluorinated carboxylic acid, CO2H, Chlorofluorocarbon, chlorine, perfluoroether, O, and sulfonic acid, SO3H. Electrochemical fluorination, Electrofluorination is the predominant method for PFC production. Due to their chemical stability, some of these perfluorinated compounds Bioaccumulation, bioaccumulate. Applications One class of perfluorinated compounds, the fluorosurfactants, are widely used in the production of teflon (PTFE) and related fluorinated polymers. They also have been used to confer hydrophobicity and stain-resistance to fabrics. They are components of Firefighting foam, fire-fighting foam. Fluorosurfactants (PFAS) reduce surface tension by concentrating at the liqui ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uncoupling Agent
An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transport chain. The result is that the cell or mitochondrion expends energy to generate a proton-motive force, but the proton-motive force is dissipated before the ATP synthase can recapture this energy and use it to make Adenosine triphosphate, ATP. Because the intracellular supply of protons is replenished, uncouplers actually stimulate cellular metabolism and oxygen consumption (despite their inhibitory effects on oxidative phosphorylation) and increase the energy cost of generating ATP. Uncouplers are capable of transporting protons through mitochondrial and lipid membranes. Description Classical uncouplers have five properties: # the complete release of respiratory control # the substitution of all coupled processes (ATP synthesis, trans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384â322 BCE), Theophrastus (â287 BCE), and Pliny the Elder (23/24â79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, JÄ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |