|
3,4-Dimethoxyamphetamine
3,4-Dimethoxyamphetamine (3,4-DMA), or simply dimethoxyamphetamine (DMA), is a psychedelic drug of the phenethylamine and amphetamine families. It is one of the dimethoxyamphetamine (DMA) series of positional isomers. The drug has been assessed in various biochemical and preclinical studies. It has been tried in humans at doses of up to 700mg intravenously, with mescaline-like effects reported. 3,4-DMA is also orally active and has produced sympathomimetic effects at a dose of 160mg. Its duration of action is unknown. Its affinity (Ki) for the rat serotonin 5-HT2A receptor has been assessed and was found to be 43,300nM. For comparison, the affinity of ''para''-methoxyamphetamine (PMA) was 33,600nM, of 2,5-dimethoxyamphetamine (2,5-DMA) was 5,200nM, and of 2,5-dimethoxy-4-methylamphetamine (DOM) was 100nM in the same study. 3,4-DMA also showed affinity for the 5-HT1 receptor (Ki = 64,600nM). The drug has additionally been found to be a monoamine oxidase inhibitor (MAOI), wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethoxyamphetamine
Dimethoxyamphetamine (DMA) is a series of six lesser-known psychedelic drugs similar in structure to the three isomers of methoxyamphetamine and six isomers of trimethoxyamphetamine. The isomers are 2,3-DMA, 2,4-DMA, 2,5-DMA, 2,6-DMA, 3,4-DMA, and 3,5-DMA. Three of the isomers were characterized by Alexander Shulgin in his book ''PiHKAL''. Little is known about their dangers or toxicity. Pharmacology 3,4-DMA, 2,4-DMA, 2,5-DMA, and their ''N''-methyl analogues all fail to produce stimulus generalization to dextroamphetamine in rodent drug discrimination tests, suggesting that they lack psychostimulant- or amphetamine-like effects, at least in rodents. This is in contrast to 2-methoxyamphetamine (OMA), 3-methoxyamphetamine (MMA), and 4-methoxyamphetamine (PMA), which all produce substitution for dextroampehtamine, albeit with far lower potency than amphetamine, methamphetamine, and other stimulants. Positional isomers 2,4-DMA * ''Dosage'': 60 mg or greater * ''Du ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-methoxy-4-hydroxyamphetamine
4-Hydroxy-3-methoxyamphetamine (HMA), also known as 3-''O''-methyl-α-methyldopamine, is an active metabolite of 3,4-methylenedioxymethamphetamine (MDMA). It is substantially less potent than MDMA or 3,4-methylenedioxyamphetamine (MDA) as a monoamine releasing agent ''in vitro''. Nonetheless, HMA has been found to induce the release of serotonin, norepinephrine, and dopamine with values of 897nM, 694nM, and 1450–3423nM, respectively, and hence acts as a lower-potency serotonin–norepinephrine–dopamine releasing agent (SNDRA). The predicted log P of HMA is 0.6. See also * 4-Hydroxy-3-methoxymethamphetamine (HMMA) * 3,4-Dihydroxyamphetamine (HHA; α-methyldopamine) * 3,4-Dihydroxymethamphetamine (HHMA; α-methylepinine) * 2,4,5-Trihydroxyamphetamine (THA) * 2,4,5-Trihydroxymethamphetamine (THMA) * 3,4-Dimethoxyamphetamine 3,4-Dimethoxyamphetamine (3,4-DMA), or simply dimethoxyamphetamine (DMA), is a psychedelic drug of the phenethylamine and amphetamine families. It i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Amphetamine
Substituted amphetamines, or simply amphetamines, are a chemical class, class of compounds based upon the amphetamine structure; it includes all derivative (chemistry), derivative compounds which are formed by replacing, or substitution reaction, substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, Empathogen-entactogen, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, tranylcypromine, bupropion, methoxyphenamine, selegiline, amfepramone, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and 2,5-dimethoxy-4-methylamphetamine, DOM (STP). Some of amphetamine's substituted Derivative (chemistry), derivatives occur in nature, for example in the leaves of ''Ephedra (genus), Ephedra'' and khat plants. Amphetamine w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonergic Psychedelic
Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary mental states (known as psychedelic experiences or "trips") and a perceived "expansion of consciousness". Also referred to as classic hallucinogens or serotonergic hallucinogens, the term ''psychedelic'' is sometimes used more broadly to include various other types of hallucinogens as well, such as those which are atypical or adjacent to psychedelia like salvia and MDMA, respectively. Classic psychedelics generally cause specific psychological, visual, and auditory changes, and oftentimes a substantially altered state of consciousness. They have had the largest influence on science and culture, and include mescaline, LSD, psilocybin, and DMT. There are a large number of both naturally occurring and synthetic serotonergic psychedelics. Most psychedelic drugs fall into one of the three families of chemical compounds: tryptamines, phenethylamines, or lysergamides. They pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2A Receptor
The 5-HT2A receptor is a subtype of the 5-HT2 receptor, 5-HT2 receptor that belongs to the serotonin receptor family and functions as a GPCR, G protein-coupled receptor (GPCR). It is a cell surface receptor that activates multiple intracellular signalling cascades. Like all 5-HT2 receptors, the 5-HT2A receptor is coupled to the Gq protein, Gq/G11 signaling pathway. It is the primary excitatory receptor subtype among the serotonin-responsive GPCRs. The 5-HT2A receptor was initially noted for its central role as the primary target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. It later regained research prominence when found to mediate, at least in part, the effects of many antipsychotic drugs, particularly atypical antipsychotic, atypical antipsychotics. Downregulation of post-synaptic 5-HT2A receptors is an adaptive response triggered by chronic administration of selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics. Elevated 5-HT2A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amphetamine
Amphetamine (contracted from Alpha and beta carbon, alpha-methylphenethylamine, methylphenethylamine) is a central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity; it is also used to treat binge eating disorder in the form of its inactive prodrug lisdexamfetamine. Amphetamine was discovered as a chemical in 1887 by Lazăr Edeleanu, and then as a drug in the late 1920s. It exists as two enantiomers: levoamphetamine and dextroamphetamine. ''Amphetamine'' properly refers to a specific chemical, the Racemic mixture, racemic free base, which is equal parts of the two enantiomers in their pure amine forms. The term is frequently used informally to refer to any combination of the enantiomers, or to either of them alone. Historically, it has been used to treat nasal congestion and depression. Amphetamine is also used as an Performance-enhancing substance, athletic performance enhancer and Nootropic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychostimulant
Stimulants (also known as central nervous system stimulants, or psychostimulants, or colloquially as uppers) are a class of drugs that increase alertness. They are used for various purposes, such as enhancing attention, motivation, cognition, mood, and physical performance. Some stimulants occur naturally, while others are exclusively synthetic. Common stimulants include caffeine, nicotine, amphetamines, cocaine, methylphenidate, and modafinil. Stimulants may be subject to varying forms of regulation, or outright prohibition, depending on jurisdiction. Stimulants increase activity in the sympathetic nervous system, either directly or indirectly. Prototypical stimulants increase synaptic concentrations of excitatory neurotransmitters, particularly norepinephrine and dopamine (e.g., methylphenidate). Other stimulants work by binding to the receptors of excitatory neurotransmitters (e.g., nicotine) or by blocking the activity of endogenous agents that promote sleep (e.g., ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Discrimination
Drug discrimination (DD) is a technique in behavioral neuroscience used to evaluate the discriminative stimulus properties or interoceptive cues of psychoactive drugs. In drug discrimination, a subject is trained on a training drug, and then it is tested with novel drugs to see if the novel drugs are experienced as similar to the training drug. In essence, the drug discrimination paradigm has the subject "tell" the experimenter "I think you gave me the training drug" or "I don't think you gave me anything". The discriminative stimulus properties of drugs are believed to reflect their subjective effects. When partial or full stimulus generalization of a test drug to a training drug occurs, the test drug can be assumed to have effects that are subjectively similar to those of the training drug. Drug discrimination tests are usually performed in animals, but have also been conducted in humans. Drug discrimination assays have been employed to assess whether drugs have stimulant- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dextroamphetamine
Dextroamphetamine (international nonproprietary name, INN: dexamfetamine) is a potent central nervous system (CNS) stimulant and enantiomer of amphetamine that is used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. It is also used illicitly to enhance Nootropic, cognitive and athletic Performance-enhancing substance, performance, and recreationally as an aphrodisiac and euphoriant. Dextroamphetamine is generally regarded as the prototype drug, prototypical stimulant. The amphetamine molecule exists as two enantiomers, levoamphetamine and dextroamphetamine. Dextroamphetamine is the Levorotation and dextrorotation, dextrorotatory, or 'right-handed', enantiomer and exhibits more pronounced effects on the central nervous system than levoamphetamine. Pharmaceutical dextroamphetamine sulfate is available as both a brand name and generic drug in a variety of dosage forms. Dextroamphetamine is sometimes prescribed as the inactive prodrug lisdexamfet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase B
Monoamine oxidase B (MAO-B) is an enzyme that in humans is encoded by the ''MAOB'' gene. The protein encoded by this gene belongs to the flavin monoamine oxidase family. It is an enzyme located in the outer mitochondrial membrane. It catalyzes the oxidative deamination of biogenic and xenobiotic amines and plays an important role in the catabolism of neuroactive and vasoactive amines in the central nervous system and peripheral tissues. This protein preferentially degrades benzylamine and phenethylamine. Similar to monoamine oxidase A (MAO-A), MAO-B is also involved in the catabolism of dopamine. Structure and function MAO-B has a hydrophobic bipartite elongated cavity that (for the "open" conformation) occupies a combined volume close to 700 Å3. hMAO-A has a single cavity that exhibits a rounder shape and is larger in volume than the "substrate cavity" of hMAO-B. The first cavity of hMAO-B has been termed the ''entrance cavity'' (290 Å3), the second ''substrate cavity'' o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase A
Monoamine oxidase A, also known as MAO-A, is an enzyme ( E.C. 1.4.3.4) that in humans is encoded by the ''MAOA'' gene. This gene is one of two neighboring gene family members that encode mitochondrial enzymes which catalyze the oxidative deamination of amines, such as norepinephrine, serotonin and tyramine. A mutation of this gene results in Brunner syndrome. This gene has also been associated with a variety of other psychiatric disorders, including antisocial behavior. Alternatively spliced transcript variants encoding multiple isoforms have been observed. Structures Gene Monoamine oxidase A, also known as MAO-A, is an enzyme that in humans is encoded by the ''MAOA'' gene. The promoter of ''MAOA'' contains conserved binding sites for Sp1, GATA2, and TBP. This gene is adjacent to a related gene ('' MAOB'') on the opposite strand of the X chromosome. In humans, there is a 30-base repeat sequence repeated several different numbers of times in the promoter re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
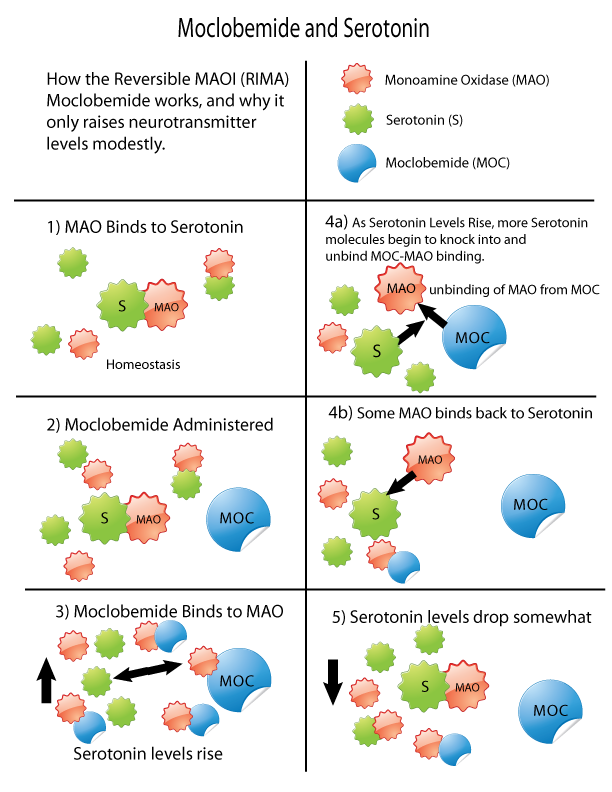
Monoamine Oxidase Inhibitor
Monoamine oxidase inhibitors (MAOIs) are a drug class, class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that binding selectivity, selectively and Enzyme inhibitor#Reversible inhibitors, reversibly enzyme inhibitor, inhibit the MAO-A enzyme. RIMAs are used clinically in the medication, treatment of major depressive disorder, depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |